Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab
- PMID: 38541760
- PMCID: PMC10971211
- DOI: 10.3390/jcm13061534
Sustained Disease Control in DME Patients upon Treatment Cessation with Brolucizumab
Abstract
Background: Treatment cessation due to a dry retina has not been systematically addressed in diabetic macular edema (DME). In three out of four patients receiving 6 mg of brolucizumab in the KITE study, treatment was terminated after the study ended. Methods: The KITE study was a double-masked, multicenter, active-controlled, randomized trial (NCT03481660) in DME patients. Per protocol, patients received five loading injections of Brolucizumab at 6-week intervals, with the option to adjust to 8 weeks in case of disease activity or to extend in the second year to a maximum of 16 weeks in the absence of retinal fluid. Results: After two years, one patient required eight weekly injections, while three patients reached a maximal treatment interval of 16 weeks. The severity of diabetic retinopathy improved in all patients with no dye leakage according to fluorescein angiography (FA) and no retinal fluid according to OCT in three patients. Treatment was paused in these three patients for >36 months, while the fourth patient required continuous treatment at 5-week intervals after switching to other licensed anti-VEGF agents. Conclusions: The adoption of treatment according to individual needs, including considering treatment cessation, may contribute to improved treatment adherence in many patients and be more frequently possible than expected.
Keywords: KITE&KESTREL studies; anti-VEGF agents; brolucizumab; case series; diabetic macular edema; intravitreal therapy; treatment cessation.
Conflict of interest statement
The authors declare that there are no conflicts of interest. J.G.G. has contributed as a principal investigator to the KITE trial, acts as an advisor and speaker for several pharmaceutical companies, and contributes to several international industry-sponsored clinical studies in the fields of retinal disease and uveitis (AbbVie, Bayer, Novartis, Roche). This manuscript is independent of these activities. None of the authors received direct or indirect support for this study, nor did they have conflicting interests with the data that are presented herein.
Figures


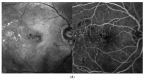


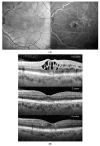
Similar articles
-
KESTREL and KITE: 52-Week Results From Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema.Am J Ophthalmol. 2022 Jun;238:157-172. doi: 10.1016/j.ajo.2022.01.004. Epub 2022 Jan 14. Am J Ophthalmol. 2022. PMID: 35038415 Clinical Trial.
-
A Randomized, Double-Masked, Multicenter, Phase III Study Assessing the Efficacy and Safety of Brolucizumab versus Aflibercept in Patients with Visual Impairment due to Diabetic Macular Edema (KITE).Klin Monbl Augenheilkd. 2020 Apr;237(4):450-453. doi: 10.1055/a-1101-9126. Epub 2020 Mar 4. Klin Monbl Augenheilkd. 2020. PMID: 32131127 Clinical Trial. English.
-
Retinal arterial occlusive vasculitis after multiple intravitreal brolucizumab injections for diabetic macular edema.Am J Ophthalmol Case Rep. 2022 Dec 30;29:101788. doi: 10.1016/j.ajoc.2022.101788. eCollection 2023 Mar. Am J Ophthalmol Case Rep. 2022. PMID: 36632338 Free PMC article.
-
Brolucizumab for the treatment of diabetic macular edema.Curr Opin Ophthalmol. 2022 May 1;33(3):167-173. doi: 10.1097/ICU.0000000000000849. Epub 2022 Mar 9. Curr Opin Ophthalmol. 2022. PMID: 35266896 Review.
-
Intravitreal steroids for macular edema in diabetes.Cochrane Database Syst Rev. 2020 Nov 17;11(11):CD005656. doi: 10.1002/14651858.CD005656.pub3. Cochrane Database Syst Rev. 2020. PMID: 33206392 Free PMC article.
Cited by
-
Treatment Cessation in Patients with Diabetic Maculopathy under Intravitreal Anti-VEGF Therapy Following a Treat-and-Extend Protocol.Ophthalmol Sci. 2025 Jun 2;5(6):100838. doi: 10.1016/j.xops.2025.100838. eCollection 2025 Nov-Dec. Ophthalmol Sci. 2025. PMID: 40689259 Free PMC article.
References
-
- Jansen M.E., Krambeer C.J., Kermany D.S., Waters J.N., Tie W., Bahadorani S., Singer J., Comstock J.M., Wannamaker K.W., Singer M.A., et al. Appointment Compliance in Patients with Diabetic Macular Edema and Exudative Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2018;49:186–190. doi: 10.3928/23258160-20180221-06. - DOI - PubMed
-
- Bressler S.B., Odia I., Glassman A.R., Danis R.P., Grover S., Hampton G.R., Jampol L.M., Maguire M.G., Melia M. Changes in Diabetic Retinopathy Severity When Treating Diabetic Macular Edema with Ranibizumab: DRCR.net Protocol I 5-Year Report. Retina. 2018;38:1896–1904. doi: 10.1097/IAE.0000000000002302. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

