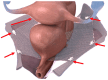
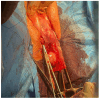
Acellular Dermal Matrices as an New Alternative for Treatment in Reproductive Organ Static Disorders: A Pilot Study
- PMID: 38541776
- PMCID: PMC10970872
- DOI: 10.3390/jcm13061550
Acellular Dermal Matrices as an New Alternative for Treatment in Reproductive Organ Static Disorders: A Pilot Study
Abstract
Background: The study aimed to evaluate the clinical effects of utilizing acellular dermal matrix (ADM) for treating pelvic organ prolapse. The motivation behind exploring a new treatment method stems from the limited efficacy of current surgical options, which are often associated with side effects. Methods: Ten patients with reproductive organ prolapse underwent surgery at the Chair and Department of Gynecology, Obstetrics, and Gynecological Oncology in Katowice. ADM was used as a support material, with eight patients receiving double TOT and two undergoing a six-point fixation mesh procedure. Pelvic organ prolapse was evaluated pre-operatively and one month post-surgery using the Pelvic Organ Prolapse Quantification (POP-Q) System. General medical history and complaints were assessed using the short form (PFDIQ-SF20). The study included ten patients aged 39 to 71 (mean: 63.6 years), all with a history of at least one vaginal delivery (mean of two). None had undergone a cesarean section. Four patients exhibited POP-Q 3, and five had POP-Q 2. Results: The mean PFDIQ-SF20 score before surgery was 70.6 points. No major complications occurred during or after surgery. One patient experienced a vaginal fungal infection and an allergic reaction to sutures. Post-operation, ailments reduced by an average of 60.76 points, with five patients reporting no complaints. Conclusions: ADM emerges as a material of interest for gynecological surgery, with initial reports highlighting its effectiveness and optimistic safety profile. Further research is warranted to explore its potential as a promising option in pelvic organ prolapse treatment.
Keywords: acellular dermal matrix; allogenic implant; pelvic organ prolapse; polypropylene mesh; static disorders.
Conflict of interest statement
The authors declare no conflicts of interest, indicating transparency and ethical conduct in the research.
Figures
References
-
- Cannone F.G., Cormaci L., Ettore C., Gulino F.A., Incognito G.G., Benvenuto D., Ettore G. Rate of Vaginal Cuff Dehiscence When Using Vicryl (Poliglactyn 910) Compared to PDS (Polydioxanone) for Vaginal Cuff Closure in Laparoscopic Hysterectomy. Medicina. 2024;60:90. doi: 10.3390/medicina60010090. - DOI - PMC - PubMed
-
- Barcz E. Uroginekologia. Schorzenia DNA Miednicy. Via Medica; Gdańsk, Poland: 2017.
LinkOut - more resources
Full Text Sources