The Efficacy and Safety of Sacubitril/Valsartan Compared to Valsartan in Patients with Heart Failure and Mildly Reduced and Preserved Ejection Fractions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 38541798
- PMCID: PMC10971386
- DOI: 10.3390/jcm13061572
The Efficacy and Safety of Sacubitril/Valsartan Compared to Valsartan in Patients with Heart Failure and Mildly Reduced and Preserved Ejection Fractions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Background: Sacubitril/valsartan improves heart failure (HF) outcomes in patients with heart failure with reduced ejection fraction (HFrEF). However, randomized controlled trials (RCTs) in patients with heart failure and mildly reduced ejection fraction (HFmrEF) and heart failure with preserved ejection fraction (HFpEF) have shown inconsistent results. We conducted this meta-analysis to comprehensively evaluate the efficacy and safety of sacubitril/valsartan compared to valsartan within this specific patient population. Methods: We searched the MEDLINE database and ClinicalTrials.gov and identified four RCTs that could be included in our analysis, with 3375 patients in the sacubitril/valsartan group and 3362 in the valsartan group. Results: Our study shows that, in patients with HFmrEF and HFpEF, sacubitril/valsartan was superior to valsartan in some of the key HF outcomes, such as the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ CSS), with a small but significant mean difference of 1.13 (95% confidence interval or CI of 0.15 to 2.11, p-value 0.024), an improvement in the New York Heart Association (NYHA) class (odds ratio or OR of 1.32, 95% CI 1.11 to 1.58, p-value 0.002), and the composite outcome of hospitalizations for HF and cardiovascular death, with a relative risk (RR) of 0.86 (95% CI 0.75 to 0.99, p-value 0.04). However, there was no additional benefit with sacubitril/valsartan compared to valsartan for the outcomes of cardiovascular death and all-cause mortality. In terms of side effects, sacubitril/valsartan was associated with a higher risk of hypotension when compared to valsartan (OR 1.67, 95% CI 1.27 to 2.19, p-value < 0.0001), but did not show an increased risk of hyperkalemia or worsening renal function. Conclusions: In individuals with HFmrEF or HFpEF, sacubitril/valsartan can result in improvements in the HF outcomes of the KCCQ CSS, the NYHA class, and the composite outcome of hospitalization for HF and cardiovascular death when compared to valsartan. While there was a higher risk of hypotension with sacubitril/valsartan compared to valsartan, there was no corresponding increase in the risk of hyperkalemia or worsening renal function.
Keywords: ARNI; HFmrEF; HFpEF; angiotensin receptor-neprilysin inhibitor; heart failure; heart failure with mildly reduced ejection fraction; heart failure with preserved ejection fraction; sacubitril/valsartan; valsartan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






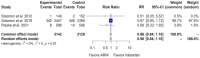
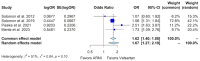


References
-
- Wilkinson I.B., McEniery C.M., Bongaerts K.H., MacCallum H., Webb D.J., Cockcroft J.R. Adrenomedullin (ADM) in the human forearm vascular bed: Effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP) Br. J. Clin. Pharmacol. 2001;52:159–164. doi: 10.1046/j.0306-5251.2001.1420.x. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

