Scoring Abdominal Symptoms in People with Cystic Fibrosis
- PMID: 38541878
- PMCID: PMC10971656
- DOI: 10.3390/jcm13061650
Scoring Abdominal Symptoms in People with Cystic Fibrosis
Abstract
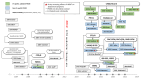
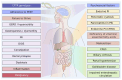
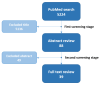
(1) Background: The introduction of highly effective CFTR-modulating therapies (HEMT) has changed the course of the disease for many people with Cystic Fibrosis (pwCF). Attention previously focused on life-threatening conditions of the respiratory system has broadened, bringing the involvement of the digestive system into the clinical and scientific focus. This emphasized the need for sensitive tools to capture and quantify changes in abdominal symptoms (AS), ideally applying patient-reported outcome measures (PROMs). (2) Methods: The present review focuses on studies addressing AS assessment deriving from the multi-organic abdominal involvement in pwCF. Among 5224 publications retrieved until Nov. 2022, 88 were eligible, and 39 were finally included. (3) Results: The review reveals that for a long time, especially before HEMT availability, AS in pwCF were assessed by single questions on abdominal complaints or non-validated questionnaires. PROMs focusing on quality of life (QOL) including a few GI-related questions were applied. Likewise, PROMs developed and partially validated for other non-CF GI pathologies, such as chronic inflammatory bowel diseases, irritable bowel syndrome, gastroesophageal reflux, constipation, or pancreatitis, were implemented. (4) Conclusions: Only lately, CF-specific GI-PROMs have been developed and validated following FDA guidelines, showing high sensitivity to changes and capturing marked and statistically significant reductions in the burden of AS achieved with HEMT implementation.
Keywords: CFAbd-Score; CFTR; PAC-QOL; PAC-SYM; PAGI-SYM; PedsQL-GI; gastrointestinal; modulator; patient-reported outcome measure (PROM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Dynamics of abdominal symptoms during the start of a new therapy with elexacaftor/tezacaftor/ivacaftor using the novel CFAbd-day2day questionnaire.Front Pharmacol. 2023 Oct 25;14:1167407. doi: 10.3389/fphar.2023.1167407. eCollection 2023. Front Pharmacol. 2023. PMID: 38026920 Free PMC article.
-
Multicenter prospective study showing a high gastrointestinal symptom burden in cystic fibrosis.J Cyst Fibros. 2023 Mar;22(2):266-274. doi: 10.1016/j.jcf.2022.10.006. Epub 2022 Oct 29. J Cyst Fibros. 2023. PMID: 36319569 Free PMC article.
-
Estimating minimal clinically important difference (MCID) for gastrointestinal symptoms in cystic fibrosis.J Cyst Fibros. 2024 Sep;23(5):991-999. doi: 10.1016/j.jcf.2024.07.013. Epub 2024 Jul 23. J Cyst Fibros. 2024. PMID: 39048465
-
Cystic fibrosis and fat malabsorption: Pathophysiology of the cystic fibrosis gastrointestinal tract and the impact of highly effective CFTR modulator therapy.Nutr Clin Pract. 2024 Apr;39 Suppl 1:S57-S77. doi: 10.1002/ncp.11122. Nutr Clin Pract. 2024. PMID: 38429959 Review.
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
Cited by
-
Disorders of Gut-Brain Interaction in a Pediatric Cystic Fibrosis Cohort.Pediatr Pulmonol. 2025 Jul;60(7):e71214. doi: 10.1002/ppul.71214. Pediatr Pulmonol. 2025. PMID: 40693578 Free PMC article.
References
-
- Cystic Fibrosis Mutation Database. [(accessed on 16 February 2023)]. Available online: http://www.genet.sickkids.on.ca/app.
-
- CFTR2 CFTR2 Variant List History. [(accessed on 16 February 2023)]. Available online: https://cftr2.org/
-
- Tabori H., Arnold C., Jaudszus A., Mentzel H.J., Renz D.M., Reinsch S., Lorenz M., Michl R., Gerber A., Lehmann T., et al. Abdominal symptoms in cystic fibrosis and their relation to genotype, history, clinical and laboratory findings. PLoS ONE. 2017;12:e0174463. doi: 10.1371/journal.pone.0174463. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

