A Short-Term Enteral Nutrition Protocol for Management of Adult Crohn's Disease-A Pilot Trial
- PMID: 38541888
- PMCID: PMC10971121
- DOI: 10.3390/jcm13061663
A Short-Term Enteral Nutrition Protocol for Management of Adult Crohn's Disease-A Pilot Trial
Abstract
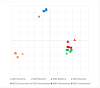
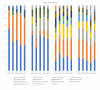
Crohn's disease (CD) is often treated with either exclusive or supplemental enteral nutrition (EN) in pediatrics, but adult practice guidelines primarily focus on medications. Here, we demonstrate the feasibility of a 4-week semi-elemental-formula-based oral nutrition delivery program for managing adult CD (n = 4). Patients consumed ~66% of calories from the formula, a finding that might provide an improved calorie target for future trials. We identified Flavinofractor as the only differentially abundant genus, distinguishing post-intervention samples from pre-intervention samples. Findings from this pilot trial demonstrate the feasibility of a partial enteral nutrition protocol in adult CD management and contribute to the growing body of literature on the potential role of EN therapy in adults with CD.
Keywords: Crohn’s disease; diet; microbiome; partial enteral nutrition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn's disease: results of a prospective cohort study.Eur J Pediatr. 2020 Mar;179(3):431-438. doi: 10.1007/s00431-019-03520-7. Epub 2019 Nov 28. Eur J Pediatr. 2020. PMID: 31781933
-
The Crohn's disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn's disease (CDED-AD): an open-label, pilot, randomised trial.Lancet Gastroenterol Hepatol. 2022 Jan;7(1):49-59. doi: 10.1016/S2468-1253(21)00299-5. Epub 2021 Nov 2. Lancet Gastroenterol Hepatol. 2022. PMID: 34739863 Clinical Trial.
-
Treatment of Active Crohn's Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn's Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes.Nutrients. 2023 Nov 4;15(21):4676. doi: 10.3390/nu15214676. Nutrients. 2023. PMID: 37960328 Free PMC article.
-
Review of exclusive enteral therapy in adult Crohn's disease.BMJ Open Gastroenterol. 2021 Sep;8(1):e000745. doi: 10.1136/bmjgast-2021-000745. BMJ Open Gastroenterol. 2021. PMID: 34580154 Free PMC article. Review.
-
Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn's disease: a meta-analysis.World J Pediatr. 2019 Feb;15(1):26-36. doi: 10.1007/s12519-018-0204-0. Epub 2019 Jan 21. World J Pediatr. 2019. PMID: 30666565 Free PMC article. Review.
References
-
- Dahlhamer J.M., Zammitti E.P., Ward B.W., Wheaton A.G., Croft J.B. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥ 18 Years—United States, 2015. Volume 65. US Department of Health and Human Services/Centers for Disease Control and Prevention; Washington, DC, USA: 2016. pp. 1166–1169. Morbidity and Mortality Weekly Report. - PubMed
-
- Sigall-Boneh R., Levine A., Lomer M., Wierdsma N., Allan P., Fiorino G., Gatti S., Jonkers D., Kierkuś J., Katsanos K.H., et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO] J. Crohn’s Colitis. 2017;11:1407–1419. doi: 10.1093/ecco-jcc/jjx109. - DOI - PubMed
-
- Lamb C.A., Kennedy N.A., Raine T., Hendy P.A., Smith P.J., Limdi J.K., Hayee B., Lomer M.C.E., Parkes G.C., Selinger C., et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68((Suppl. 3)):s1–s106. doi: 10.1136/gutjnl-2019-318484. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

