Circulating Tumor DNA (ctDNA) Clearance May Predict Treatment Response in Neoadjuvant Colorectal Cancer Management
- PMID: 38541909
- PMCID: PMC10970814
- DOI: 10.3390/jcm13061684
Circulating Tumor DNA (ctDNA) Clearance May Predict Treatment Response in Neoadjuvant Colorectal Cancer Management
Abstract
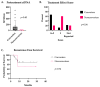
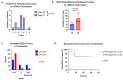
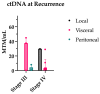
Background: Circulating tumor DNA (ctDNA) is extracellular DNA released by tumors and has been proposed as a marker of residual disease as well as a predictor of disease recurrence in the adjuvant setting. However, data are lacking on the utility of this biomarker in the neoadjuvant setting. Methods: We performed a retrospective study of stage III and IV colorectal cancer patients receiving neoadjuvant treatment at a single institution. Results: Seventeen patients converted from a positive pre-neoadjuvant ctDNA to a negative ctDNA prior to surgery. Five patients remained persistently positive despite systemic treatment. ctDNA conversion was found to be associated with a higher incidence of favorable treatment effect scores on final surgical pathology. There was no difference in recurrence-free survival in this small population. Furthermore, no added benefit was identified for patients receiving additional neoadjuvant therapy after the time of positive to negative ctDNA conversion. Conclusions: This study highlights the potential utility of ctDNA and the need for prospective trials in the neoadjuvant setting to monitor treatment response and guide decisions on treatment duration.
Keywords: circulating tumor DNA (ctDNA); colorectal cancer; neoadjuvant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ohara S., Suda K., Sakai K., Nishino M., Chiba M., Shimoji M., Takemoto T., Fujino T., Koga T., Hamada A., et al. Prognostic implications of preoperative versus postoperative circulating tumor DNA in surgically resected lung cancer patients: A pilot study. Transl. Lung Cancer Res. 2020;9:1915–1923. doi: 10.21037/tlcr-20-505. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

