Cardiac Sarcoidosis-Diagnostic and Therapeutic Challenges
- PMID: 38541919
- PMCID: PMC10970915
- DOI: 10.3390/jcm13061694
Cardiac Sarcoidosis-Diagnostic and Therapeutic Challenges
Abstract
Sarcoidosis is a multisystem disorder of unknown etiology. The leading hypothesis involves an antigen-triggered dysregulated T-cell-driven immunologic response leading to non-necrotic granulomas. In cardiac sarcoidosis (CS), the inflammatory response can lead to fibrosis, culminating in clinical manifestations such as atrioventricular block and ventricular arrhythmias. Cardiac manifestations frequently present as first and isolated signs or may appear in conjunction with extracardiac manifestations. The incidence of sudden cardiac death (SCD) is high. Diagnosis remains a challenge. For a definite diagnosis, endomyocardial biopsy (EMB) is suggested. In clinical practice, compatible findings in advanced imaging using cardiovascular magnetic resonance (CMR) and/or positron emission tomography (PET) in combination with extracardiac histological proof is considered sufficient. Management revolves around the control of myocardial inflammation by employing immunosuppression. However, data regarding efficacy are merely based on observational evidence. Prevention of SCD is of particular importance and several guidelines provide recommendations regarding device therapy. In patients with manifest CS, outcome data indicate a 5-year survival of around 90% and a 10-year survival in the range of 80%. Data for patients with silent CS are conflicting; some studies suggest an overall benign course of disease while others reported contrasting observations. Future research challenges involve better understanding of the immunologic pathogenesis of the disease for a targeted therapy, improving imaging to aid early diagnosis, assessing the need for screening of asymptomatic patients and randomized trials.
Keywords: cardiac sarcoidosis; cardiovascular magnetic resonance; implantable cardioverter–defibrillator; inflammatory cardiomyopathy; positron emission tomography; sudden cardiac death; ventricular arrhythmia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
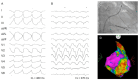
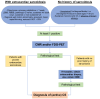


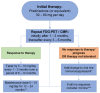

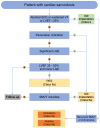
References
-
- Kandolin R., Lehtonen J., Airaksinen J., Vihinen T., Miettinen H., Ylitalo K., Kaikkonen K., Tuohinen S., Haataja P., Kerola T., et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. - DOI - PubMed
-
- Zeppenfeld K., Tfelt-Hansen J., de Riva M., Winkel B.G., Behr E.R., Blom N.A., Charron P., Corrado D., Dagres N., de Chillou C., et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. - DOI - PubMed
-
- Könemann H., Dagres N., Merino J.L., Sticherling C., Zeppenfeld K., Tfelt-Hansen J., Eckardt L. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace. 2023;25:euad091. doi: 10.1093/europace/euad091. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

