Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption
- PMID: 38542057
- PMCID: PMC10969889
- DOI: 10.3390/ijms25063083
Timeframe Analysis of Novel Synthetic Cannabinoids Effects: A Study on Behavioral Response and Endogenous Cannabinoids Disruption
Abstract
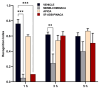
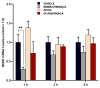
This study investigates the impact of SCs consumption by assessing the effects of three novel synthetic cannabinoids (SCs); MDMB-CHMINACA, 5F-ADB-PINACA, and APICA post-drug treatment. SCs are known for their rapid onset (<1 min) and prolonged duration (≥5 h). Therefore, this research aimed to assess behavioral responses and their correlation with endocannabinoids (ECs) accumulation in the hippocampus, and EC's metabolic enzymes alteration at different timeframes (1-3-5-h) following drug administration. Different extents of locomotive disruption and sustained anxiety-like symptoms were observed throughout all-encompassing timeframes of drug administration. Notably, MDMB-CHMINACA induced significant memory impairment at 1 and 3 h. Elevated levels of anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) were detected 1 h post-MDMB-CHMINACA and 5F-ADB-PINACA administration. Reduced mRNA expression levels of fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MAGL) (AEA and 2-AG degrading enzymes, respectively), and brain-derived neurotrophic factor (BDNF) occurred at 1 h, with FAAH levels remaining reduced at 3 h. These findings suggest a connection between increased EC content and decreased BDNF expression following SC exposure. Cognitive disruption, particularly motor coordination decline and progressive loss manifested in a time-dependent manner across all the analyzed SCs. Our study highlights the importance of adopting a temporal framework when assessing the effects of SCs.
Keywords: 5F-ADB-PINACA; APICA; JWH-018; MDMB-CHMINACA; behavior; indazole/indole–carboxamide; locomotive; memory.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Synthesis and in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA.ACS Chem Neurosci. 2020 Dec 16;11(24):4434-4446. doi: 10.1021/acschemneuro.0c00644. Epub 2020 Nov 30. ACS Chem Neurosci. 2020. PMID: 33253529
-
Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review.Biology (Basel). 2022 May 23;11(5):796. doi: 10.3390/biology11050796. Biology (Basel). 2022. PMID: 35625524 Free PMC article. Review.
-
Cannabinoid-like effects of five novel carboxamide synthetic cannabinoids.Neurotoxicology. 2019 Jan;70:72-79. doi: 10.1016/j.neuro.2018.11.004. Epub 2018 Nov 12. Neurotoxicology. 2019. PMID: 30439379 Free PMC article.
-
Elevation of endocannabinoids in the brain by synthetic cannabinoid JWH-018: mechanism and effect on learning and memory.Sci Rep. 2019 Jul 3;9(1):9621. doi: 10.1038/s41598-019-45969-4. Sci Rep. 2019. PMID: 31270353 Free PMC article.
-
Motor vehicle collisions caused by the 'super-strength' synthetic cannabinoids, MAM-2201, 5F-PB-22, 5F-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014.Forensic Toxicol. 2017;35(2):244-251. doi: 10.1007/s11419-017-0369-6. Epub 2017 May 29. Forensic Toxicol. 2017. PMID: 28706566 Free PMC article. Review.
Cited by
-
Assessment of Abuse Potential of Three Indazole-Carboxamide Synthetic Cannabinoids 5F-ADB, MDMB-4en-PINACA and ADB-4en-PINACA.Int J Mol Sci. 2025 Jul 3;26(13):6409. doi: 10.3390/ijms26136409. Int J Mol Sci. 2025. PMID: 40650186 Free PMC article.
References
-
- Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

