PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase
- PMID: 38542085
- PMCID: PMC10970662
- DOI: 10.3390/ijms25063111
PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase
Abstract
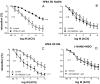
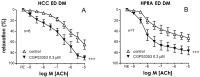
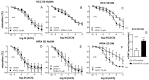
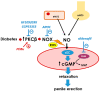
Erectile dysfunction (ED) is a frequent and difficult-to-treat condition in diabetic men. Protein kinase C (PKC) is involved in diabetes-related vascular and cavernosal alterations. We aimed to evaluate the role of PKC in endothelial dysfunction and NO/cGMP impairment associated with diabetic ED in the human corpus cavernosum (CC) and penile resistance arteries (PRAs) and the potential mechanisms involved. Functional responses were determined in the CC and PRAs in patients with non-diabetic ED and diabetic ED undergoing penile prosthesis insertion. PKC activator 12,13-phorbol-dibutyrate (PDBu) impaired endothelial relaxations and cGMP generation in response to acetylcholine in the CC from non-diabetic ED. PDBu also impaired responses to a PDE5 inhibitor, sildenafil, in non-diabetic ED patients. Conversely, a PKC inhibitor, GF109203X, improved endothelial, neurogenic, and PDE5-inhibitor-induced relaxations and cGMP generation only in the CC in diabetic ED patients. Endothelial and PDE5-inhibitor-induced vasodilations of PRAs were potentiated only in diabetes. Improvements in endothelial function in diabetes were also achieved with a specific inhibitor of the PKCβ2 isoform or an NADPH-oxidase inhibitor, apocynin, which prevented PDBu-induced impairment in non-diabetic patients. PKC inhibition counteracted NO/cGMP impairment and endothelial dysfunction in diabetes-related ED, potentially improving response to PDE5 inhibition.
Keywords: NADPH oxidase; NO/cGMP pathway; diabetes; endothelial dysfunction; erectile dysfunction; human corpus cavernosum; human penile arteries; protein kinase C.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

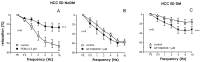

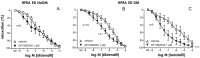
Similar articles
-
Nebivolol potentiates the efficacy of PDE5 inhibitors to relax corpus cavernosum and penile arteries from diabetic patients by enhancing the NO/cGMP pathway.J Sex Med. 2014 May;11(5):1182-92. doi: 10.1111/jsm.12477. J Sex Med. 2014. PMID: 24877179
-
STIM/Orai Inhibition as a Strategy for Alleviating Diabetic Erectile Dysfunction Through Modulation of Rat and Human Penile Tissue Contractility and in vivo Potentiation of Erectile Responses.J Sex Med. 2022 Dec;19(12):1733-1749. doi: 10.1016/j.jsxm.2022.08.200. Epub 2022 Oct 1. J Sex Med. 2022. PMID: 36195535
-
Ca2+ -activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction.Br J Pharmacol. 2013 May;169(2):449-61. doi: 10.1111/bph.12143. Br J Pharmacol. 2013. PMID: 23441682 Free PMC article.
-
Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction.Urology. 2002 Sep;60(2 Suppl 2):4-11. doi: 10.1016/s0090-4295(02)01686-2. Urology. 2002. PMID: 12414329 Review.
-
New treatment options for erectile dysfunction in patients with diabetes mellitus.Drugs. 2004;64(23):2667-88. doi: 10.2165/00003495-200464230-00004. Drugs. 2004. PMID: 15537369 Review.
Cited by
-
Targeting TRPC-5 Channel Inhibition to Improve Penile Vascular Function in Erectile Dysfunction.Int J Mol Sci. 2025 Feb 8;26(4):1431. doi: 10.3390/ijms26041431. Int J Mol Sci. 2025. PMID: 40003900 Free PMC article.
-
Omega-3 fatty acids improves tamoxifen-induced sexual dysfunction in male Wistar rats by modulating NO/cGMP signaling and monoamine neurotransmitters activities.Psychopharmacology (Berl). 2025 Jun 18. doi: 10.1007/s00213-025-06836-5. Online ahead of print. Psychopharmacology (Berl). 2025. PMID: 40528104
-
Dimethyl Fumarate Improves Diabetic Erectile Dysfunction in Rats via Nrf2-Mediated Suppression of Penile Endothelial Oxidative Stress.Reprod Sci. 2025 Aug 18. doi: 10.1007/s43032-025-01956-x. Online ahead of print. Reprod Sci. 2025. PMID: 40826215
References
-
- Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. - DOI - PubMed
-
- Ma J.X., Wang B., Li H.S., Yu J., Hu H.M., Ding C.F., Chen W.Q. Uncovering the Mechanisms of Leech and Centipede Granules in the Treatment of Diabetes Mellitus-Induced Erectile Dysfunction Utilising Network Pharmacology. J. Ethnopharmacol. 2021;265:113358. doi: 10.1016/j.jep.2020.113358. - DOI - PubMed
-
- Angulo J., González-Corrochano R., Cuevas P., Fernández A., La Fuente J.M., Rolo F., Allona A., Sáenz de Tejada I. Diabetes Exacerbates the Functional Deficiency of NO/CGMP Pathway Associated with Erectile Dysfunction in Human Corpus Cavernosum and Penile Arteries. J. Sex. Med. 2010;7:758–768. doi: 10.1111/j.1743-6109.2009.01587.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

