The Role of Gut Microbiota in Neuromyelitis Optica Spectrum Disorder
- PMID: 38542152
- PMCID: PMC10970017
- DOI: 10.3390/ijms25063179
The Role of Gut Microbiota in Neuromyelitis Optica Spectrum Disorder
Abstract
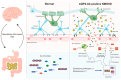
Neuromyelitis optica spectrum disorder (NMOSD) is a rare, disabling inflammatory disease of the central nervous system (CNS). Aquaporin-4 (AQP4)-specific T cells play a key role in the pathogenesis of NMOSD. In addition to immune factors, T cells recognizing the AQP4 epitope showed cross-reactivity with homologous peptide sequences in C. perfringens proteins, suggesting that the gut microbiota plays an integral role in the pathogenicity of NMOSD. In this review, we summarize research on the involvement of the gut microbiota in the pathophysiology of NMOSD and its possible pathogenic mechanisms. Among them, Clostridium perfringens and Streptococcus have been confirmed to play a role by multiple studies. Based on this evidence, metabolites produced by gut microbes, such as short-chain fatty acids (SCFAs), tryptophan (Trp), and bile acid (BA) metabolites, have also been found to affect immune cell metabolism. Therefore, the role of the gut microbiota in the pathophysiology of NMOSD is very important. Alterations in the composition of the gut microbiota can lead to pathological changes and alter the formation of microbiota-derived components and metabolites. It can serve as a biomarker for disease onset and progression and as a potential disease-modifying therapy.
Keywords: gut microbiota; metabolites; neuromyelitis optica spectrum disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., de Seze J., Fujihara K., Greenberg B., Jacob A., et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

