Polyphenol-Rich Extract from 'Limoncella' Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage
- PMID: 38542183
- PMCID: PMC10969867
- DOI: 10.3390/ijms25063210
Polyphenol-Rich Extract from 'Limoncella' Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage
Abstract
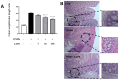
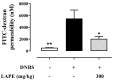
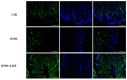
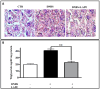
Inflammatory bowel conditions can involve nearly all organ systems and induce pathological processes through increased oxidative stress, lipid peroxidation and disruption of the immune response. Patients with inflammatory bowel disease (IBD) are at high risk of having extra-intestinal manifestations, for example, in the hepatobiliary system. In 30% of patients with IBD, the blood values of liver enzymes, such as AST and ALT, are increased. Moreover, treatments for inflammatory bowel diseases may cause liver toxicity. Apple polyphenol extracts are widely acknowledged for their potential antioxidant effects, which help prevent damage from oxidative stress, reduce inflammation, provide protection to the liver, and enhance lipid metabolism. The aim of this study was to investigate whether the polyphenol apple extract from Malus domestica cv. 'Limoncella' (LAPE) may be an effective intervention for the treatment of IBD-induced hepatotoxicity. The LAPE was administrated in vivo by oral gavage (3-300 mg/kg) once a day for 3 consecutive days, starting 24 h after the induction of dinitro-benzenesulfonic acid (DNBS) colitis in mice. The results showed that LAPE significantly attenuated histological bowel injury, myeloperoxidase activity, tumor necrosis factor and interleukin (IL-1β) expressions. Furthermore, LAPE significantly improved the serum lipid peroxidation and liver injury in DNBS-induced colitis, as well as reduced the nuclear transcription factor-kappaB activation. In conclusion, these results suggest that LAPE, through its antioxidant and anti-inflammatory properties, could prevent liver damage induced by inflammatory bowel disease.
Keywords: antioxidant; apple polyphenols; inflammatory bowel disease; liver inflammation; nutraceuticals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Costa C., Tsatsakis A., Mamoulakis C., Teodoro M., Briguglio G., Caruso E., Tsoukalas D., Margina D., Dardiotis E., Kouretas D., et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017;110:286–299. doi: 10.1016/j.fct.2017.10.023. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

