The Role of Neutral Sphingomyelinase-2 (NSM2) in the Control of Neutral Lipid Storage in T Cells
- PMID: 38542220
- PMCID: PMC10970209
- DOI: 10.3390/ijms25063247
The Role of Neutral Sphingomyelinase-2 (NSM2) in the Control of Neutral Lipid Storage in T Cells
Abstract
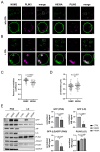
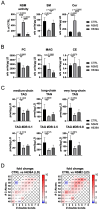
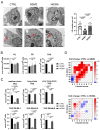
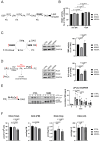
The accumulation of lipid droplets (LDs) and ceramides (Cer) is linked to non-alcoholic fatty liver disease (NAFLD), regularly co-existing with type 2 diabetes and decreased immune function. Chronic inflammation and increased disease severity in viral infections are the hallmarks of the obesity-related immunopathology. The upregulation of neutral sphingomyelinase-2 (NSM2) has shown to be associated with the pathology of obesity in tissues. Nevertheless, the role of sphingolipids and specifically of NSM2 in the regulation of immune cell response to a fatty acid (FA) rich environment is poorly studied. Here, we identified the presence of the LD marker protein perilipin 3 (PLIN3) in the intracellular nano-environment of NSM2 using the ascorbate peroxidase APEX2-catalyzed proximity-dependent biotin labeling method. In line with this, super-resolution structured illumination microscopy (SIM) shows NSM2 and PLIN3 co-localization in LD organelles in the presence of increased extracellular concentrations of oleic acid (OA). Furthermore, the association of enzymatically active NSM2 with isolated LDs correlates with increased Cer levels in these lipid storage organelles. NSM2 enzymatic activity is not required for NSM2 association with LDs, but negatively affects the LD numbers and cellular accumulation of long-chain unsaturated triacylglycerol (TAG) species. Concurrently, NSM2 expression promotes mitochondrial respiration and fatty acid oxidation (FAO) in response to increased OA levels, thereby shifting cells to a high energetic state. Importantly, endogenous NSM2 activity is crucial for primary human CD4+ T cell survival and proliferation in a FA rich environment. To conclude, our study shows a novel NSM2 intracellular localization to LDs and the role of enzymatically active NSM2 in metabolic response to enhanced FA concentrations in T cells.
Keywords: cholesteryl ester (CE); diacylglycerol (DAG); fatty acid oxidation (FAO); lipid droplet (LD); monounsaturated fatty acid (MUFA); neutral sphingomyelinase-2 (NSM2); plasma membrane (PM); triacylglycerol (TAG).
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Role of Neutral Sphingomyelinase-2 (NSM 2) in the Control of T Cell Plasma Membrane Lipid Composition and Cholesterol Homeostasis.Front Cell Dev Biol. 2019 Oct 15;7:226. doi: 10.3389/fcell.2019.00226. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31681760 Free PMC article.
-
Decoration of myocellular lipid droplets with perilipins as a marker for in vivo lipid droplet dynamics: A super-resolution microscopy study in trained athletes and insulin resistant individuals.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Feb;1866(2):158852. doi: 10.1016/j.bbalip.2020.158852. Epub 2020 Nov 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33160079
-
A unifying mathematical model of lipid droplet metabolism reveals key molecular players in the development of hepatic steatosis.FEBS J. 2017 Oct;284(19):3245-3261. doi: 10.1111/febs.14189. Epub 2017 Sep 6. FEBS J. 2017. PMID: 28763157
-
Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD.Mol Metab. 2021 Aug;50:101115. doi: 10.1016/j.molmet.2020.101115. Epub 2020 Nov 10. Mol Metab. 2021. PMID: 33186758 Free PMC article. Review.
-
Pathophysiology of lipid droplet proteins in liver diseases.Exp Cell Res. 2016 Jan 15;340(2):187-92. doi: 10.1016/j.yexcr.2015.10.021. Epub 2015 Oct 26. Exp Cell Res. 2016. PMID: 26515554 Free PMC article. Review.
Cited by
-
Dynamic changes in the proximitome of neutral sphingomyelinase-2 (nSMase2) in TNFα stimulated Jurkat cells.Front Immunol. 2024 Jul 9;15:1435701. doi: 10.3389/fimmu.2024.1435701. eCollection 2024. Front Immunol. 2024. PMID: 39044828 Free PMC article.
-
Ceramide homeostasis in hepatic lipid droplets.Biochem Soc Trans. 2025 Apr 18;53(2):509-518. doi: 10.1042/BST20253042. Biochem Soc Trans. 2025. PMID: 40605341 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

