Trimetazidine Improves Mitochondrial Dysfunction in SOD1G93A Cellular Models of Amyotrophic Lateral Sclerosis through Autophagy Activation
- PMID: 38542223
- PMCID: PMC10970050
- DOI: 10.3390/ijms25063251
Trimetazidine Improves Mitochondrial Dysfunction in SOD1G93A Cellular Models of Amyotrophic Lateral Sclerosis through Autophagy Activation
Abstract
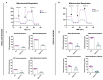
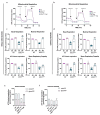
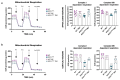
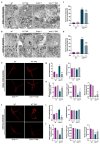
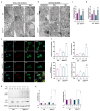
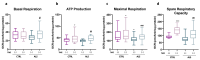
Amyotrophic Lateral Sclerosis (ALS) is considered the prototype of motor neuron disease, characterized by motor neuron loss and muscle waste. A well-established pathogenic hallmark of ALS is mitochondrial failure, leading to bioenergetic deficits. So far, pharmacological interventions for the disease have proven ineffective. Trimetazidine (TMZ) is described as a metabolic modulator acting on different cellular pathways. Its efficacy in enhancing muscular and cardiovascular performance has been widely described, although its molecular target remains elusive. We addressed the molecular mechanisms underlying TMZ action on neuronal experimental paradigms. To this aim, we treated murine SOD1G93A-model-derived primary cultures of cortical and spinal enriched motor neurons, as well as a murine motor-neuron-like cell line overexpressing SOD1G93A, with TMZ. We first characterized the bioenergetic profile of the cell cultures, demonstrating significant mitochondrial dysfunction that is reversed by acute TMZ treatments. We then investigated the effect of TMZ in promoting autophagy processes and its impact on mitochondrial morphology. Finally, we demonstrated the effectiveness of TMZ in terms of the mitochondrial functionality of ALS-rpatient-derived peripheral blood mononuclear cells (PBMCs). In summary, our results emphasize the concept that targeting mitochondrial dysfunction may represent an effective therapeutic strategy for ALS. The findings demonstrate that TMZ enhances mitochondrial performance in motor neuron cells by activating autophagy processes, particularly mitophagy. Although further investigations are needed to elucidate the precise molecular pathways involved, these results hold critical implications for the development of more effective and specific derivatives of TMZ for ALS treatment.
Keywords: Amyotrophic Lateral Sclerosis; SOD1G93A; Trimetazidine; autophagy; mitochondria; spinal and cortical primary cultures.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Repurposing of Trimetazidine for amyotrophic lateral sclerosis: A study in SOD1G93A mice.Br J Pharmacol. 2022 Apr;179(8):1732-1752. doi: 10.1111/bph.15738. Epub 2022 Jan 13. Br J Pharmacol. 2022. PMID: 34783031 Free PMC article.
-
OPTN gene therapy increases autophagy and protects mitochondria in SOD1-G93A-expressing transgenic mice and cells.FEBS J. 2024 Feb;291(4):795-813. doi: 10.1111/febs.17009. Epub 2023 Dec 1. FEBS J. 2024. PMID: 37983563
-
ALS-associated mutation SOD1G93A leads to abnormal mitochondrial dynamics in osteocytes.Bone. 2018 Jan;106:126-138. doi: 10.1016/j.bone.2017.10.010. Epub 2017 Oct 10. Bone. 2018. PMID: 29030231 Free PMC article.
-
Dysregulated mitochondrial Ca2+ and ROS signaling in skeletal muscle of ALS mouse model.Arch Biochem Biophys. 2019 Mar 15;663:249-258. doi: 10.1016/j.abb.2019.01.024. Epub 2019 Jan 22. Arch Biochem Biophys. 2019. PMID: 30682329 Free PMC article. Review.
-
Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis.Cells. 2024 Jan 29;13(3):248. doi: 10.3390/cells13030248. Cells. 2024. PMID: 38334639 Free PMC article. Review.
Cited by
-
Brown Adipose Tissue undergoes pathological perturbations and shapes C2C12 myoblast homeostasis in the SOD1-G93A mouse model of Amyotrophic Lateral Sclerosis.Heliyon. 2025 Jan 23;11(3):e41801. doi: 10.1016/j.heliyon.2025.e41801. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39916853 Free PMC article.
-
Mitigating the Functional Deficit after Neurotoxic Motoneuronal Loss by an Inhibitor of Mitochondrial Fission.Int J Mol Sci. 2024 Jun 27;25(13):7059. doi: 10.3390/ijms25137059. Int J Mol Sci. 2024. PMID: 39000168 Free PMC article.
References
-
- Levi Montalcini R., Feldman E.L., Goutman S.A., Hardiman O., Al-Chalabi A., Chió A., Savelieff M.G., Kiernan M.C. Amyotrophic Lateral Sclerosis 2 Recent Advances in the Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. Ser. Lancet Neurol. 2022;21:480–493. doi: 10.1016/S1474-4422(21)00465-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CNR IFT DBA.AD005.225 -NUTRAGE- FOE2021/National Research Council
- RF-2019-12369105/Ministero della Salute
- HyperALS/Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica
- 'Unraveling the protective effects of NOS1AP in the rescue of TDP-43 loss of function pathological mechanisms/Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica
- Ref. 27019/Italian Association for Cancer Research
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

