Multilayer Electrospun Scaffolds of Opposite-Charged Chitosans
- PMID: 38542232
- PMCID: PMC10969954
- DOI: 10.3390/ijms25063256
Multilayer Electrospun Scaffolds of Opposite-Charged Chitosans
Abstract
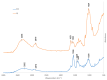
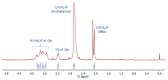
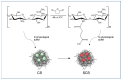
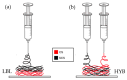
Chitosan (CS) is a polysaccharide obtainable by the deacetylation of chitin, which is highly available in nature and is consequently low-cost. Chitosan is already used in the biomedical field (e.g., guides for nerve reconstruction) and has been proposed as a biomaterial for tissue regeneration in different body districts, including bone tissue. The interest in chitosan as a biomaterial stems from its ease of functionalization due to the presence of reactive groups, its antibacterial properties, its ease of processing to obtain porous matrices, and its inherent similarity to polysaccharides that constitute the human extracellular matrix, such as hyaluronic acid (HA). Here, chitosan was made to react with succinic anhydride to develop a negatively charged chitosan (SCS) that better mimics HA. FT-IR and NMR analyses confirmed the presence of the carboxylic groups in the modified polymer. Four different electrospun matrices were prepared: CS, SCS, a layer-by-layer matrix (LBL), and a matrix with both CS and SCS simultaneously electrospun (HYB). All the matrices containing SCS showed increased human osteoblast proliferation, mineralization, and gene expression, with the best results obtained with HYB compared to the control (CS). Moreover, the antibacterial potential of CS was preserved in all the SCS-containing matrices, and the pure SCS matrix demonstrated a significant reduction in bacterial proliferation of both S. aureus and E. coli.
Keywords: E. coli; S. aureus; bone tissue engineering; electrospinning; multilayer matrices; negatively charged chitosan; osteoblasts; succinyl chitosan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. doi: 10.1016/S1381-5148(00)00038-9. - DOI
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
-
- Yilmaz Atay H. Antibacterial Activity of Chitosan-Based Systems. In: Jana S., Jana S., editors. Functional Chitosan. Springer Singapore; Singapore: 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

