A Water-Based Biocoating to Increase the Infection Resistance and Osteoconductivity of Titanium Surfaces
- PMID: 38542241
- PMCID: PMC10969944
- DOI: 10.3390/ijms25063267
A Water-Based Biocoating to Increase the Infection Resistance and Osteoconductivity of Titanium Surfaces
Abstract
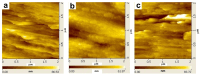
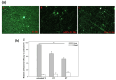
As the population ages, the number of patients undergoing total hip arthroplasty (THA) and total knee arthroplasty (TKA) continues to increase. Infections after primary arthroplasty are rare but have high rates of morbidity and mortality, as well as enormous financial implications for healthcare systems. Numerous methods including the use of superhydrophobic coatings, the incorporation of antibacterial agents, and the application of topographical treatments have been developed to reduce bacterial attachment to medical devices. However, most of these methods require complex manufacturing processes. Thus, the main purpose of this study was to apply biocoatings to titanium (Ti) surfaces to increase their infection resistance and osteoconductivity via simple processes, without organic reagents. We modified titanium surfaces with a combination of aminomalononitrile (AMN) and an antibiotic-loaded mesoporous bioactive glass (MBG) and evaluated both the antibacterial effects of the coating layer and its effect on osteoblast proliferation and differentiation. The properties of the modified surface, such as the hydrophilicity, roughness, and surface morphology, were characterized via contact angle measurements, atomic force microscopy, and scanning electron microscopy. The cell proliferation reagent WST-1 assay and the alkaline phosphatase (ALP) assay were used to determine the degrees of adhesion and differentiation, respectively, of the MG-63 osteoblast-like cells on the surface. Antimicrobial activity was evaluated by examining the survival rate and inhibition zone of Escherichia coli (E. coli). The AMN coating layer reduced the water contact angle (WCA) of the titanium surface from 87° ± 2.5° to 53° ± 2.3° and this change was retained even after immersion in deionized water for five weeks, demonstrating the stability of the AMN coating. Compared with nontreated titanium and polydopamine (PDA) coating layers, the AMN surface coating increased MG-63 cell attachment, spreading, and early ALP expression; reduced E. coli adhesion; and increased the percentage of dead bacteria. In addition, the AMN coating served as an adhesion layer for the subsequent deposition of MBG-containing antibiotic nanoparticles. The synergistic effects of the AMN layer and antibiotics released from the MBG resulted in an obvious E. coli inhibition zone that was not observed in the nontreated titanium group.
Keywords: antibacterial; biocoating; osteoconductivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Jin X., Gallego Luxan B., Hanly M., Pratt N.L., Harris I., de Steiger R., Graves S.E., Jorm L. Estimating incidence rates of periprosthetic joint infection after hip and knee arthroplasty for osteoarthritis using linked registry and administrative health data. Bone Jt. J. 2022;104-B:1060–1066. doi: 10.1302/0301-620X.104B9.BJJ-2022-0116.R1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

