Strigolactones Might Regulate Ovule Development after Fertilization in Xanthoceras sorbifolium
- PMID: 38542248
- PMCID: PMC10969979
- DOI: 10.3390/ijms25063276
Strigolactones Might Regulate Ovule Development after Fertilization in Xanthoceras sorbifolium
Abstract
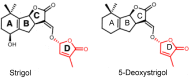
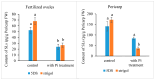
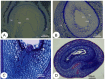
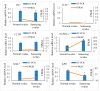
Strigolactones (SLs) were recently defined as a novel class of plant hormones that act as key regulators of diverse developmental processes and environmental responses. Much research has focused on SL biosynthesis and signaling in roots and shoots, but little is known about whether SLs are produced in early developing seeds and about their roles in ovule development after fertilization. This study revealed that the fertilized ovules and early developing pericarp in Xanthoceras sorbifolium produced minute amounts of two strigolactones: 5-deoxystrigol and strigol. Their content decreased in the plants with the addition of exogenous phosphate (Pi) compared to those without the Pi treatment. The exogenous application of an SL analog (GR24) and a specific inhibitor of SL biosynthesis (TIS108) affected early seed development and fruit set. In the Xanthoceras genome, we identified 69 potential homologs of genes involved in SL biological synthesis and signaling. Using RNA-seq to characterize the expression of these genes in the fertilized ovules, 37 genes were found to express differently in the fertilized ovules that were aborting compared to the normally developing ovules. A transcriptome analysis also revealed that in normally developing ovules after fertilization, 12 potential invertase genes were actively expressed. Hexoses (glucose and fructose) accumulated at high concentrations in normally developing ovules during syncytial endosperm development. In contrast, a low ratio of hexose and sucrose levels was detected in aborting ovules with a high strigolactone content. XsD14 virus-induced gene silencing (VIGS) increased the hexose content in fertilized ovules and induced the proliferation of endosperm free nuclei, thereby promoting early seed development and fruit set. We propose that the crosstalk between sugar and strigolactone signals may be an important part of a system that accurately regulates the abortion of ovules after fertilization. This study is useful for understanding the mechanisms underlying ovule abortion, which will serve as a guide for genetic or chemical approaches to promote seed yield in Xanthoceras.
Keywords: Xanthoceras sorbifolium; fertilized ovules; invertase; strigolactone signals; sugar.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

