Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities
- PMID: 38542285
- PMCID: PMC10970165
- DOI: 10.3390/ijms25063311
Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities
Abstract
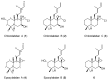
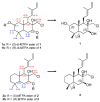
Chemical investigation of the ethyl acetate (EtOAc) extract from a marine-derived actinomycete, Streptomyces griseorubens, resulted in the discovery of five new labdane-type diterpenoids: chlorolabdans A-C (1-3), epoxylabdans A and B (4 and 5), along with one known analog (6). The structures of the new compounds were determined by spectroscopic analysis (HR-ESIMS, 1D, and 2D NMR) and by comparing their experimental data with those in the literature. The new compounds were evaluated for their antimicrobial activity, and 2 displayed significant activity against Gram-positive bacteria, with minimum inhibitory concentration (MIC) values ranging from 4 to 8 µg/mL. Additionally, 1, 2, and 4 were tested for their cytotoxicity against seven blood cancer cell lines by CellTiter-Glo (CTG) assay and six solid cancer cell lines by sulforhodamine B (SRB) assay; 1, 2, and 4 exhibited cytotoxic activities against some blood cancer cell lines, with concentration causing 50% cell growth inhibition (IC50) values ranging from 1.2 to 22.5 µM.
Keywords: Streptomyces griseorubens; antimicrobial; chlorolabdan; cytotoxicity; diterpenoids; epoxylabdan; labdane.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
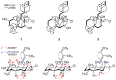
Similar articles
-
Pyrrole-Containing Alkaloids from a Marine-Derived Actinobacterium Streptomyces zhaozhouensis and Their Antimicrobial and Cytotoxic Activities.Mar Drugs. 2023 Mar 6;21(3):167. doi: 10.3390/md21030167. Mar Drugs. 2023. PMID: 36976216 Free PMC article.
-
Geliboluols A-D: Kaurane-Type Diterpenoids from the Marine-Derived Rare Actinomycete Actinomadura geliboluensis.Mar Drugs. 2025 Feb 10;23(2):78. doi: 10.3390/md23020078. Mar Drugs. 2025. PMID: 39997202 Free PMC article.
-
Labdane Diterpenoids from Salvia leriifolia: Absolute Configuration, Antimicrobial and Cytotoxic Activities.Planta Med. 2016 Sep;82(14):1279-85. doi: 10.1055/s-0042-107798. Epub 2016 Jun 9. Planta Med. 2016. PMID: 27280932
-
Violapyrones H and I, new cytotoxic compounds isolated from Streptomyces sp. associated with the marine starfish Acanthaster planci.Mar Drugs. 2014 May 30;12(6):3283-91. doi: 10.3390/md12063283. Mar Drugs. 2014. PMID: 24886866 Free PMC article.
-
Structural Characterization and Biological Evaluation of 18-Nor-ent-labdane Diterpenoids from Grazielia gaudichaudeana.Chem Biodivers. 2019 May;16(5):e1800644. doi: 10.1002/cbdv.201800644. Epub 2019 Apr 25. Chem Biodivers. 2019. PMID: 30843651
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

