Molecular Pharmacology of Gelsemium Alkaloids on Inhibitory Receptors
- PMID: 38542362
- PMCID: PMC10970644
- DOI: 10.3390/ijms25063390
Molecular Pharmacology of Gelsemium Alkaloids on Inhibitory Receptors
Abstract
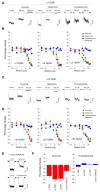
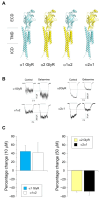
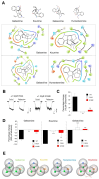
Indole alkaloids are the main bioactive molecules of the Gelsemium genus plants. Diverse reports have shown the beneficial actions of Gelsemium alkaloids on the pathological states of the central nervous system (CNS). Nevertheless, Gelsemium alkaloids are toxic for mammals. To date, the molecular targets underlying the biological actions of Gelsemium alkaloids at the CNS remain poorly defined. Functional studies have determined that gelsemine is a modulator of glycine receptors (GlyRs) and GABAA receptors (GABAARs), which are ligand-gated ion channels of the CNS. The molecular and physicochemical determinants involved in the interactions between Gelsemium alkaloids and these channels are still undefined. We used electrophysiological recordings and bioinformatic approaches to determine the pharmacological profile and the molecular interactions between koumine, gelsemine, gelsevirine, and humantenmine and these ion channels. GlyRs composed of α1 subunits were inhibited by koumine and gelsevirine (IC50 of 31.5 ± 1.7 and 40.6 ± 8.2 μM, respectively), while humantenmine did not display any detectable activity. The examination of GlyRs composed of α2 and α3 subunits showed similar results. Likewise, GABAARs were inhibited by koumine and were insensitive to humantenmine. Further assays with chimeric and mutated GlyRs showed that the extracellular domain and residues within the orthosteric site were critical for the alkaloid effects, while the pharmacophore modeling revealed the physicochemical features of the alkaloids for the functional modulation. Our study provides novel information about the molecular determinants and functional actions of four major Gelsemium indole alkaloids on inhibitory receptors, expanding our knowledge regarding the interaction of these types of compounds with protein targets of the CNS.
Keywords: GABAA receptor; Gelsemium alkaloids; bioinformatics; electrophysiology; glycine receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Peng Y., Liang J., Xue Y., Khan A., Zhang P., Feng T., Song D., Zhou Y., Wei X. Genus Gelsemium and its Endophytic Fungi—Comprehensive Review of their Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Curr. Top. Med. Chem. 2023;23:2452–2487. doi: 10.2174/1568026623666230825105233. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

