Effect of Donor Age on Endocrine Function of and Immune Response to Ovarian Grafts
- PMID: 38542404
- PMCID: PMC10970747
- DOI: 10.3390/ijms25063431
Effect of Donor Age on Endocrine Function of and Immune Response to Ovarian Grafts
Abstract
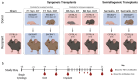
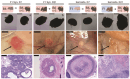
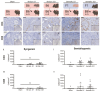
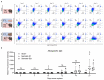
Premature loss of ovarian function (POI) is associated with numerous negative side effects, including vasomotor symptoms, sleep and mood disturbances, disrupted urinary function, and increased risks for osteoporosis and heart disease. Hormone replacement therapy (HRT), the standard of care for POI, delivers only a subset of ovarian hormones and fails to mimic the monthly cyclicity and daily pulsatility characteristic of healthy ovarian tissue in reproductive-aged individuals whose ovarian tissue contains thousands of ovarian follicles. Ovarian tissue allografts have the potential to serve as an alternative, cell-based HRT, capable of producing the full panel of ovarian hormones at physiologically relevant doses and intervals. However, the risks associated with systemic immune suppression (IS) required to prevent allograft rejection outweigh the potential benefits of comprehensive and dynamic hormone therapy. This work investigates whether the age of ovarian tissue donor animals affects the function of, and immune response to, subcutaneous ovarian grafts. We performed syngeneic and semi-allogeneic ovarian transplants using tissue from mice aged 6-8 (D7) or 20-22 (D21) days and evaluated ovarian endocrine function and immune response in a mouse model of POI. Our results revealed that tissue derived from D7 donors, containing an ample and homogeneous primordial follicle reserve, was more effective in fully restoring hypothalamic-pituitary-ovarian feedback. In contrast, tissue derived from D21 donors elicited anti-donor antibodies with higher avidity compared to tissue from younger donors, suggesting that greater immunogenicity may be a trade-off of using mature donors. This work contributes to our understanding of the criteria donor tissue must meet to effectively function as a cell-based HRT and explores the importance of donor age as a factor in ovarian allograft rejection.
Keywords: donor age; ovarian transplant; primary ovarian insufficiency.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Encapsulation of ovarian allograft precludes immune rejection and promotes restoration of endocrine function in immune-competent ovariectomized mice.Sci Rep. 2019 Nov 12;9(1):16614. doi: 10.1038/s41598-019-53075-8. Sci Rep. 2019. PMID: 31719632 Free PMC article.
-
Concentrated exosomes from menstrual blood-derived stromal cells improves ovarian activity in a rat model of premature ovarian insufficiency.Stem Cell Res Ther. 2021 Mar 12;12(1):178. doi: 10.1186/s13287-021-02255-3. Stem Cell Res Ther. 2021. PMID: 33712079 Free PMC article.
-
Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency.Hum Reprod. 2015 Mar;30(3):608-15. doi: 10.1093/humrep/deu353. Epub 2015 Jan 6. Hum Reprod. 2015. PMID: 25567618
-
Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of pre mature ovarian failure.J Reprod Immunol. 2021 Sep;147:103363. doi: 10.1016/j.jri.2021.103363. Epub 2021 Aug 20. J Reprod Immunol. 2021. PMID: 34450435 Review.
-
The Evaluation of Ovarian Function Recovery Following Treatment of Primary Ovarian Insufficiency: A Systematic Review.Front Endocrinol (Lausanne). 2022 Apr 28;13:855992. doi: 10.3389/fendo.2022.855992. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35573993 Free PMC article.
Cited by
-
Serum bisphenol S levels are associated with decreased ovarian reserve function: a single-center study.Am J Transl Res. 2024 Oct 15;16(10):5961-5969. doi: 10.62347/AQMO8416. eCollection 2024. Am J Transl Res. 2024. PMID: 39544747 Free PMC article.
References
-
- Sheshpari S., Shahnazi M., Mobarak H., Ahmadian S., Bedate A.M., Nariman-Saleh-Fam Z., Nouri M., Rahbarghazi R., Mahdipour M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019;17:396. doi: 10.1186/s12967-019-02149-2. - DOI - PMC - PubMed
-
- El Khoudary S.R., Aggarwal B., Beckie T.M., Hodis H.N., Johnson A.E., Langer R.D., Limacher M.C., Manson J.E., Stefanick M.L., Allison M.A., et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

