Molecular Determinants Involved in the Docking and Uptake of Tumor-Derived Extracellular Vesicles: Implications in Cancer
- PMID: 38542421
- PMCID: PMC10970554
- DOI: 10.3390/ijms25063449
Molecular Determinants Involved in the Docking and Uptake of Tumor-Derived Extracellular Vesicles: Implications in Cancer
Abstract
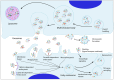
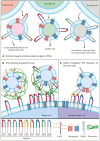
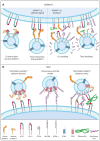
Extracellular vesicles produced by tumor cells (TEVs) influence all stages of cancer development and spread, including tumorigenesis, cancer progression, and metastasis. TEVs can trigger profound phenotypic and functional changes in target cells through three main general mechanisms: (i) docking of TEVs on target cells and triggering of intra-cellular signaling; (ii) fusion of TEVs and target cell membranes with release of TEVs molecular cargo in the cytoplasm of recipient cell; and (iii) uptake of TEVs by recipient cells. Though the overall tumor-promoting effects of TEVs as well as the general mechanisms involved in TEVs interactions with, and uptake by, recipient cells are relatively well established, current knowledge about the molecular determinants that mediate the docking and uptake of tumor-derived EVs by specific target cells is still rather deficient. These molecular determinants dictate the cell and organ tropism of TEVs and ultimately control the specificity of TEVs-promoted metastases. Here, we will review current knowledge on selected specific molecules that mediate the tropism of TEVs towards specific target cells and organs, including the integrins, ICAM-1 Inter-Cellular Adhesion Molecule), ALCAM (Activated Leukocyte Cell Adhesion Molecule), CD44, the metalloproteinases ADAM17 (A Disintegrin And Metalloproteinase member 17) and ADAM10 (A Disintegrin And Metalloproteinase member 10), and the tetraspanin CD9.
Keywords: ADAM10; ADAM17; ALCAM/CD166; CD44; CD9; ICAM-1/CD54; TEV docking; TEV uptake; adhesion receptors; integrins; tetraspanins; tumor-derived extracellular vesicles (TEVs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
ALCAM/CD166 Is Involved in the Binding and Uptake of Cancer-Derived Extracellular Vesicles.Int J Mol Sci. 2022 May 20;23(10):5753. doi: 10.3390/ijms23105753. Int J Mol Sci. 2022. PMID: 35628559 Free PMC article.
-
The capture of extracellular vesicles endogenously released by xenotransplanted tumours induces an inflammatory reaction in the premetastatic niche.J Extracell Vesicles. 2023 May;12(5):e12326. doi: 10.1002/jev2.12326. J Extracell Vesicles. 2023. PMID: 37194998 Free PMC article.
-
CD44 and Tumor-Derived Extracellular Vesicles (TEVs). Possible Gateway to Cancer Metastasis.Int J Mol Sci. 2021 Feb 2;22(3):1463. doi: 10.3390/ijms22031463. Int J Mol Sci. 2021. PMID: 33540535 Free PMC article. Review.
-
Revealing the presence of tear extracellular vesicles in Keratoconus.Exp Eye Res. 2022 Nov;224:109242. doi: 10.1016/j.exer.2022.109242. Epub 2022 Sep 6. Exp Eye Res. 2022. PMID: 36084727 Free PMC article.
-
Pushing Forward the DNA Walkers in Connection with Tumor-Derived Extracellular Vesicles.Int J Nanomedicine. 2024 Jun 19;19:6231-6252. doi: 10.2147/IJN.S464895. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38915916 Free PMC article. Review.
Cited by
-
Role of CD44-Positive Extracellular Vesicles Derived from Highly Metastatic Mouse Mammary Carcinoma Cells in Pre-Metastatic Niche Formation.Int J Mol Sci. 2024 Sep 9;25(17):9742. doi: 10.3390/ijms25179742. Int J Mol Sci. 2024. PMID: 39273689 Free PMC article.
-
Expression of ADAM10 and CD58 in Acute and Chronic Lymphocytic Leukemia: Influence of Disease Stage and Chemotherapy.Iran J Pathol. 2025 Summer;20(3):316-325. doi: 10.30699/ijp.2025.2060663.3459. Epub 2025 Jul 1. Iran J Pathol. 2025. PMID: 40746920 Free PMC article.
References
-
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

