Optical Intracranial Self-Stimulation (oICSS): A New Behavioral Model for Studying Drug Reward and Aversion in Rodents
- PMID: 38542425
- PMCID: PMC10970671
- DOI: 10.3390/ijms25063455
Optical Intracranial Self-Stimulation (oICSS): A New Behavioral Model for Studying Drug Reward and Aversion in Rodents
Abstract
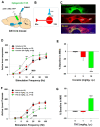
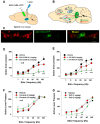
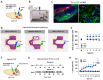
Brain-stimulation reward, also known as intracranial self-stimulation (ICSS), is a commonly used procedure for studying brain reward function and drug reward. In electrical ICSS (eICSS), an electrode is surgically implanted into the medial forebrain bundle (MFB) in the lateral hypothalamus or the ventral tegmental area (VTA) in the midbrain. Operant lever responding leads to the delivery of electrical pulse stimulation. The alteration in the stimulation frequency-lever response curve is used to evaluate the impact of pharmacological agents on brain reward function. If a test drug induces a leftward or upward shift in the eICSS response curve, it implies a reward-enhancing or abuse-like effect. Conversely, if a drug causes a rightward or downward shift in the functional response curve, it suggests a reward-attenuating or aversive effect. A significant drawback of eICSS is the lack of cellular selectivity in understanding the neural substrates underlying this behavior. Excitingly, recent advancements in optical ICSS (oICSS) have facilitated the development of at least three cell type-specific oICSS models-dopamine-, glutamate-, and GABA-dependent oICSS. In these new models, a comparable stimulation frequency-lever response curve has been established and employed to study the substrate-specific mechanisms underlying brain reward function and a drug's rewarding versus aversive effects. In this review article, we summarize recent progress in this exciting research area. The findings in oICSS have not only increased our understanding of the neural mechanisms underlying drug reward and addiction but have also introduced a novel behavioral model in preclinical medication development for treating substance use disorders.
Keywords: aversion; brain-stimulation reward (BSR); drugs of abuse; intracranial self-stimulation (ICSS); optical ICSS; optogenetics; reward.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Optogenetic brain-stimulation reward: A new procedure to re-evaluate the rewarding versus aversive effects of cannabinoids in dopamine transporter-Cre mice.Addict Biol. 2021 Jul;26(4):e13005. doi: 10.1111/adb.13005. Epub 2021 Feb 3. Addict Biol. 2021. PMID: 33538103 Free PMC article.
-
Dopamine receptor sub-types involvement in nucleus accumbens and ventral tegmentum but not in medial prefrontal cortex: on self-stimulation of lateral hypothalamus and ventral mesencephalon.Behav Brain Res. 1997 Jul;86(2):171-9. doi: 10.1016/s0166-4328(96)02263-2. Behav Brain Res. 1997. PMID: 9134152
-
Cholinergic and GABAergic modulation of self-stimulation of lateral hypothalamus and ventral tegmentum: effects of carbachol, atropine, bicuculline, and picrotoxin.Physiol Behav. 1997 Mar;61(3):411-8. doi: 10.1016/s0031-9384(96)00452-0. Physiol Behav. 1997. PMID: 9089760
-
Intracranial self-stimulation to evaluate abuse potential of drugs.Pharmacol Rev. 2014 Jul;66(3):869-917. doi: 10.1124/pr.112.007419. Pharmacol Rev. 2014. PMID: 24973197 Free PMC article. Review.
-
Determinants of opioid abuse potential: Insights using intracranial self-stimulation.Peptides. 2019 Feb;112:23-31. doi: 10.1016/j.peptides.2018.10.007. Epub 2018 Nov 1. Peptides. 2019. PMID: 30391425 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

