Blood Transcriptomics Identifies Multiple Gene Expression Pathways Associated with the Clinical Efficacy of Hymenoptera Venom Immunotherapy
- PMID: 38542470
- PMCID: PMC10971012
- DOI: 10.3390/ijms25063499
Blood Transcriptomics Identifies Multiple Gene Expression Pathways Associated with the Clinical Efficacy of Hymenoptera Venom Immunotherapy
Abstract
Allergen-specific venom immunotherapy (VIT) is a well-established therapy for Hymenoptera venom allergy (HVA). However, the precise mechanism underlying its clinical effect remains uncertain. Our study aimed to identify the molecular mechanisms associated with VIT efficiency. We prospectively included 19 patients with HVA undergoing VIT (sampled before the beginning of VIT, after reaching the maintenance dose, one year after finishing VIT, and after a sting challenge) and 9 healthy controls. RNA sequencing of whole blood was performed on an Illumina sequencing platform. Longitudinal transcriptomic profiling revealed the importance of the inhibition of the NFκB pathway and the downregulation of DUX4 transcripts for the early protection and induction of tolerance after finishing VIT. Furthermore, successful treatment was associated with inhibiting Th2, Th17, and macrophage alternative signalling pathways in synergy with the inhibition of the PPAR pathway and further silencing of the Th2 response. The immune system became activated when reaching the maintenance dose and was suppressed after finishing VIT. Finally, successful VIT restores the immune system's balance to a state similar to that of healthy individuals. Our results underline the important role of the inhibition of four pathways in the clinical effect of VIT: Th2, Th17, NFκB, and macrophage signalling. Two biomarkers specific for successful VIT, regardless of the time of sampling, were C4BPA and RPS10-NUDT3 and should be further tested as potential biomarkers.
Keywords: Hymenoptera venom immunotherapy; longitudinal transcriptomic profiling; successful venom immunotherapy; tolerance induction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
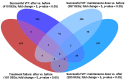
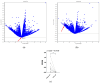


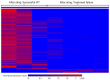

Similar articles
-
Cellular and Humoral Response After Induction of Protection and After Finishing Hymenoptera Venom Immunotherapy.Biomolecules. 2024 Nov 24;14(12):1494. doi: 10.3390/biom14121494. Biomolecules. 2024. PMID: 39766201 Free PMC article.
-
Specific Immunotherapy in Hymenoptera Venom Allergy and Concomitant Malignancy: A Retrospective Follow-up Focusing on Effectiveness and Safety.J Investig Allergol Clin Immunol. 2017;27(6):370-377. doi: 10.18176/jiaci.0184. Epub 2017 Jul 4. J Investig Allergol Clin Immunol. 2017. PMID: 28675375
-
Hymenoptera venom allergy in outdoor workers: Occupational exposure, clinical features and effects of allergen immunotherapy.Hum Vaccin Immunother. 2017 Feb;13(2):477-483. doi: 10.1080/21645515.2017.1264748. Hum Vaccin Immunother. 2017. PMID: 27924689 Free PMC article.
-
Clinical aspects of hymenoptera venom allergy and venom immunotherapy.Eur Ann Allergy Clin Immunol. 2019 Nov;51(6):244-258. doi: 10.23822/EurAnnACI.1764-1489.113. Eur Ann Allergy Clin Immunol. 2019. PMID: 31594296 Review.
-
The Lights and the Shadows of Controlled Sting Challenge With Hymenoptera.J Investig Allergol Clin Immunol. 2022 Oct 11;32(5):357-366. doi: 10.18176/jiaci.0838. Epub 2022 Jun 22. J Investig Allergol Clin Immunol. 2022. PMID: 35735250 Review.
References
-
- Lydyard P., Whelan A., Fanger M.W. Immunology. 2nd ed. Garland Science/Bios Scientific Publishers; New York, NY, USA: 2004.
-
- Sturm G.J., Varga E., Roberts G., Mosbech H., Bilò M.B., Akdis C.A., Antolín-Amérigo D., Cichocka-Jarosz E., Gawlik R., Jakob T., et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018;73:744–764. doi: 10.1111/all.13262. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

