Gold Nanoparticle-Modified Carbon-Fiber Microelectrodes for the Electrochemical Detection of Cd2+ via Fast-Scan Cyclic Voltammetry
- PMID: 38542541
- PMCID: PMC10971841
- DOI: 10.3390/mi15030294
Gold Nanoparticle-Modified Carbon-Fiber Microelectrodes for the Electrochemical Detection of Cd2+ via Fast-Scan Cyclic Voltammetry
Abstract
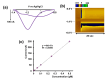
Neurotoxic heavy metals, such as Cd2+, pose a significant global health concern due to their increased environmental contamination and subsequent detrimental health hazards they pose to human beings. These metal ions can breach the blood-brain barrierblood-brain barrier, leading to severe and often irreversible damage to the central nervous system and other vital organs. Therefore, developing a highly sensitive, robust, and rapid in vivo detection method for these hazardous heavy metal ions is of the utmost importance for early detection, thus initiating timely therapeutics. Detecting ultra-low levels of toxic metal ions in vivo and obtaining accurate speciation information remains a challenge with conventional analytical techniques. In this study, we fabricated a novel carbon carbon-fiber microelectrode (CFM)-based sensor that can detect Cd2+ ions using fast-scan cyclic voltammetry by electrodepositing gold nanoparticles (AuNP). We optimized electrochemical parameters that generate a unique cyclic voltammogram (CV) of Cd2+ at a temporal resolution of 100 ms with our novel sensor. All our experiments were performed in tris buffer that mimics the artificial cerebellum fluid. We established a calibration curve resulting in a limit of detection (LOD) of 0.01 µM with a corresponding sensitivity of 418.02 nA/ µM. The sensor's selectivity was evaluated in the presence of other metal ions, and it was noteworthy to observe that the sensor retained its ability to produce the distinctive Cd2+ CV, even when the concentration of other metal ions was 200 times higher than that of Cd2+. We also found that our sensor could detect free Cd2+ ions in the presence of complexing agents. Furthermore, we analyzed the solution chemistry of each of those Cd2+-ligand solutions using a geochemical model, PHREEQC. The concentrations of free Cd2+ ions determined through our electrochemical data align well with geochemical modeling data, thus validating the response of our novel sensor. Furthermore, we reassessed our sensor's LOD in tris buffer based on the concentration of free Cd2+ ions determined through PHREEQC analysis, revealing an LOD of 0.00132 µM. We also demonstrated the capability of our sensor to detect Cd2+ ions in artificial urine samples, showcasing its potential for application in actual biological samples. To the best of our knowledge, this is the first AuNP-modified, CFM-based Cd2+ sensor capable of detecting ultra-low concentrations of free Cd2+ ions in different complex matrices, including artificial urine at a temporal resolution of 100 ms, making it an excellent analytical tool for future real-time, in vivo detection, particularly in the brain.
Keywords: cadmium; carbon carbon-fiber microelectrodes; electrodeposition; fast scan cyclic voltammetry; gold nanoparticles; real-time analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




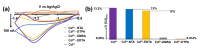
References
Grants and funding
LinkOut - more resources
Full Text Sources

