Progress in Electronic, Energy, Biomedical and Environmental Applications of Boron Nitride and MoS2 Nanostructures
- PMID: 38542596
- PMCID: PMC10972515
- DOI: 10.3390/mi15030349
Progress in Electronic, Energy, Biomedical and Environmental Applications of Boron Nitride and MoS2 Nanostructures
Abstract
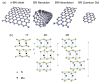
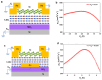
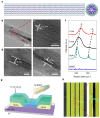
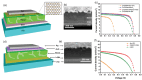
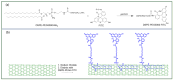
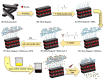
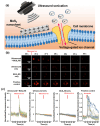
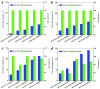
In this review, we examine recent progress using boron nitride (BN) and molybdenum disulfide (MoS2) nanostructures for electronic, energy, biomedical, and environmental applications. The scope of coverage includes zero-, one-, and two-dimensional nanostructures such as BN nanosheets, BN nanotubes, BN quantum dots, MoS2 nanosheets, and MoS2 quantum dots. These materials have sizable bandgaps, differentiating them from other metallic nanostructures or small-bandgap materials. We observed two interesting trends: (1) an increase in applications that use heterogeneous materials by combining BN and MoS2 nanostructures with other nanomaterials, and (2) strong research interest in environmental applications. Last, we encourage researchers to study how to remove nanomaterials from air, soil, and water contaminated with nanomaterials. As nanotechnology proceeds into various applications, environmental contamination is inevitable and must be addressed. Otherwise, nanomaterials will go into our food chain much like microplastics.
Keywords: biomedicine; boron nitride; electronics; energy; environment; molybdenum disulfide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

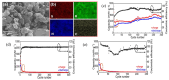

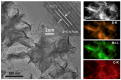
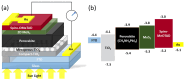
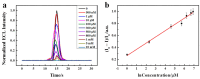




References
-
- Ekimov A.I., Onushchenko A.A. Quantum size effect in 3-dimensional microscopic semiconductor crystals. JETP Lett. 1981;34:345–349. doi: 10.1134/S0021364023130040. - DOI
-
- Brus L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984;80:4403–4409. doi: 10.1063/1.447218. - DOI
-
- Murray C.B., Norris D.J., Bawendi M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993;115:8706–8715. doi: 10.1021/ja00072a025. - DOI
-
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. - DOI
-
- Roco M.C., Williams R.S., Alivisatos P. Vision for Nanotechnology in the Next Decade. Springer; Dordrecht, The Netherlands: 2000. Nanotechnology Research Directions: IWGN Workshop Report.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

