Organosulfur Compounds in Colorectal Cancer Prevention and Progression
- PMID: 38542713
- PMCID: PMC10974587
- DOI: 10.3390/nu16060802
Organosulfur Compounds in Colorectal Cancer Prevention and Progression
Abstract
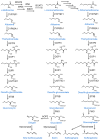
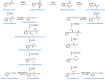
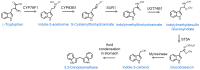
This work represents an overview of the current investigations involving organosulfur compounds and colorectal cancer. The molecules discussed in this review have been investigated regarding their impact on colorectal cancer directly, at the in vitro, in vivo, and clinical stages. Organosulfur compounds may have indirect effects on colorectal cancer, such as due to their modulating effects on the intestinal microbiota or their positive effects on intestinal mucosal health. Here, we focus on their direct effects via the repression of multidrug resistance proteins, triggering of apoptosis (via the inhibition of histone deacetylases, increases in reactive oxygen species, p53 activation, β-catenin inhibition, damage in the mitochondrial membrane, etc.), activation of TGF-β, binding to tubulin, inhibition of angiogenesis and metastasis mechanisms, and inhibition of cancer stem cells, among others. In general, the interesting positive effects of these nutraceuticals in in vitro tests must be further analyzed with more in vivo models before conducting clinical trials.
Keywords: allicin; glucosinolate; indol-3-carbinol; isothiocyanate; sulforaphane.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wild C., Weiderpass E., Stewart B.W. In: World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer. Wild C.P., Weiderpass E., Stewart B.W., editors. IARC; Lyon, France: 2020. - PubMed
-
- Murphy N., Ward H.A., Jenab M., Rothwell J.A., Boutron-Ruault M.-C., Carbonnel F., Kvaskoff M., Kaaks R., Kühn T., Boeing H., et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin. Gastroenterol. Hepatol. 2019;17:1323–1331.e6. doi: 10.1016/j.cgh.2018.07.030. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

