The Inhibitory Effect of Early Pregnancy Factor on Red Meat Neu5Gc-Mediated Antibody Production in CMAH-/- Mice
- PMID: 38542816
- PMCID: PMC10975788
- DOI: 10.3390/nu16060905
The Inhibitory Effect of Early Pregnancy Factor on Red Meat Neu5Gc-Mediated Antibody Production in CMAH-/- Mice
Abstract
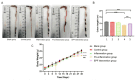
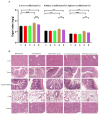
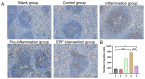
The meat derived from mammals such as cows, sheep, and pigs is commonly referred to as red meat. Recent studies have shown that consuming red meat can activate the immune system, produce antibodies, and subsequently develop into tumors and cancer. This is due to the presence of a potential carcinogenic compound in red meat called N-ethanol neuraminic acid (Neu5Gc). Neu5Gc is a common sialic monosaccharide in mammals, synthesized from N-acetylneuraminic acid (Neu5Ac) in the body and typically present in most mammals. However, due to the lack of the CMAH gene encoding the cytidine 5'-monophosphate Neu5Ac hydroxylase, humans are unable to synthesize Neu5Gc. Compared to primates such as mice or chimpanzees, the specific loss of Neu5Gc expression in humans is attributed to fixed genome mutations in CMAH. Although Neu5Gc cannot be produced, it can be introduced from specific dietary sources such as red meat and milk, so it is necessary to use mice or chimpanzees that knock out the CMAH gene instead of humans as experimental models. Further research has shown that early pregnancy factor (EPF) has the ability to regulate CD4+T cell-dependent immune responses. In this study, we established a simulated human animal model using C57/BL6 mice with CMAH gene knockout and analyzed the inhibitory effect of EPF on red meat Neu5Gc-induced CMAH-/- C57/BL6 mouse antibody production and chronic inflammation development. The results showed that the intervention of EPF reduced slow weight gain and shortened colon length in mice. In addition, EPF treatment significantly reduced the levels of anti Neu5Gc antibodies in the body, as well as the inflammatory factors IL-6 and IL-1β, TNF-α and the activity of MPO. In addition, it also alleviated damage to liver and intestinal tissues and reduced the content of CD4 cells and the expression of B cell activation molecules CD80 and CD86 in mice. In summary, EPF effectively inhibited Neu5Gc-induced antibody production, reduced inflammation levels in mice, and alleviated Neu5Gc-induced inflammation. This will provide a new re-search concept and potential approach for developing immunosuppressants to address safety issues related to long-term consumption of red meat.
Keywords: CMAH; EPF; N-glycolylneuraminic acid; Neu5Gc; early pregnancy factor; red meat.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms.Proc Natl Acad Sci U S A. 2019 Aug 6;116(32):16036-16045. doi: 10.1073/pnas.1902902116. Epub 2019 Jul 22. Proc Natl Acad Sci U S A. 2019. PMID: 31332008 Free PMC article.
-
Dietary Neu5Ac Intervention Protects Against Atherosclerosis Associated With Human-Like Neu5Gc Loss-Brief Report.Arterioscler Thromb Vasc Biol. 2021 Nov;41(11):2730-2739. doi: 10.1161/ATVBAHA.120.315280. Epub 2021 Sep 30. Arterioscler Thromb Vasc Biol. 2021. PMID: 34587757 Free PMC article.
-
Dietary intake of the red meat-derived glycan Neu5Gc fuels colorectal cancer through up-regulation of Wnt signaling pathway.Cancer Lett. 2025 Apr 28;616:217598. doi: 10.1016/j.canlet.2025.217598. Epub 2025 Feb 27. Cancer Lett. 2025. PMID: 40023392
-
An Overview of the Importance and Value of Porcine Species in Sialic Acid Research.Biology (Basel). 2022 Jun 11;11(6):903. doi: 10.3390/biology11060903. Biology (Basel). 2022. PMID: 35741423 Free PMC article. Review.
-
N-Glycolylneuraminic Acid (Neu5Gc) Null Large Animals by Targeting the CMP-Neu5Gc Hydroxylase (CMAH).Front Immunol. 2019 Oct 15;10:2396. doi: 10.3389/fimmu.2019.02396. eCollection 2019. Front Immunol. 2019. PMID: 31681287 Free PMC article. Review.
References
-
- Zhu H.C., Yang X., Zhang C., Zhu C., Tao G.Z., Zhao L.J., Tang S.W., Shu Z., Cai J., Dai S.B., et al. Red and Processed Meat Intake Is Associated with Higher Gastric Cancer Risk: A Meta-Analysis of Epidemiological Observational Studies. PLoS ONE. 2013;8:e70955. doi: 10.1371/journal.pone.0070955. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

