Exploring the Influence of Morphology on Bipolaron-Polaron Ratios and Conductivity in Polypyrrole in the Presence of Surfactants
- PMID: 38542834
- PMCID: PMC10975615
- DOI: 10.3390/molecules29061197
Exploring the Influence of Morphology on Bipolaron-Polaron Ratios and Conductivity in Polypyrrole in the Presence of Surfactants
Abstract
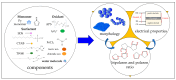
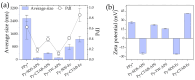
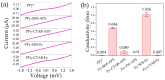
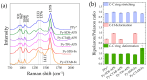
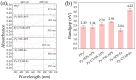
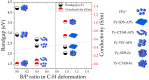
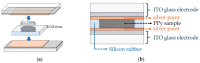
This research aims to deepen the understanding of the relationship between conductivity and morphology in polypyrrole (PPy) via a comparison of the bipolaron to polaron ratios with a focus on the C-H deformation area. PPy samples were synthesized with different surfactants: sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), and tween 80 (TW). This study revealed that SDS significantly altered the bipolaron and polaron in the C-H deformation region and showed higher conductivity than other surfactants. Notably, the morphological shifts to a sheet-like structure when using ammonium sulfate (APS) contrasted with the particle-like form observed with ferric chloride (FeCl3). These results showed that if the oxidant changed, the bipolaron and polaron ratios in C-H deformation were unrelated to PPy morphology. However, this work showed a consistent relationship between SDS use, the bipolaron and polaron ratios in the C-H deformation, and the conductivity properties. Moreover, the natural positive charge of PPy and negatively charged SDS molecules may lead to an electrostatic interaction between PPy and SDS. This work assumes that this interaction might cause the transformation of polaron to bipolaron in the C-H deformation region, resulting in improved conductivity of PPy. This work offers more support for the future investigation of PPy characteristics.
Keywords: C-H deformation; Raman spectra; bipolaron and polaron ratio; polypyrrole; sodium dodecyl sulfate; surfactant.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Study of Chemical Polymerization of Polypyrrole with SDS Soft Template: Physical, Chemical, and Electrical Properties.ACS Omega. 2023 Dec 13;8(51):48946-48957. doi: 10.1021/acsomega.3c06511. eCollection 2023 Dec 26. ACS Omega. 2023. PMID: 38162777 Free PMC article.
-
Polaron and bipolaron induced charge carrier transportation for enhanced photocatalytic H2 production.Nanoscale. 2020 Jul 14;12(26):14213-14221. doi: 10.1039/d0nr02950e. Epub 2020 Jul 1. Nanoscale. 2020. PMID: 32608424
-
Synthesis and conductive properties of polypyrrole nanocomposites.J Nanosci Nanotechnol. 2011 Nov;11(11):9836-9. doi: 10.1166/jnn.2011.5219. J Nanosci Nanotechnol. 2011. PMID: 22413305
-
Synergistic effects in the gas sensitivity of polypyrrole/single wall carbon nanotube composites.Sensors (Basel). 2012;12(6):7965-74. doi: 10.3390/s120607965. Epub 2012 Jun 8. Sensors (Basel). 2012. PMID: 22969381 Free PMC article.
-
Recent advances of polypyrrole conducting polymer film for biomedical application: Toward a viable platform for cell-microbial interactions.Adv Colloid Interface Sci. 2023 Apr;314:102860. doi: 10.1016/j.cis.2023.102860. Epub 2023 Feb 16. Adv Colloid Interface Sci. 2023. PMID: 36931199 Review.
Cited by
-
Nanofibrous Membranes Based on Collagen and Conductive Polymers with Perspective for Biological Applications.Membranes (Basel). 2025 Jun 11;15(6):177. doi: 10.3390/membranes15060177. Membranes (Basel). 2025. PMID: 40559356 Free PMC article.
References
-
- Wen J., Tian Y., Mei Z., Wu W., Tian Y. Synthesis of Polypyrrole Nanoparticles and Their Applications in Electrically Conductive Adhesives for Improving Conductivity. RSC Adv. 2017;7:53219–53225. doi: 10.1039/C7RA09725E. - DOI
-
- Xing S., Zhao G. Morphology, Structure, and Conductivity of Polypyrrole Prepared in the Presence of Mixed Surfactants in Aqueous Solutions. J. Appl. Polym. Sci. 2007;104:1987–1996. doi: 10.1002/app.25912. - DOI
-
- Kausaite-Minkstimiene A., Mazeiko V., Ramanaviciene A., Ramanavicius A. Evaluation of Chemical Synthesis of Polypyrrole Particles. Colloids Surf. Physicochem. Eng. Asp. 2015;483:224–231. doi: 10.1016/j.colsurfa.2015.05.008. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

