Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite
- PMID: 38542837
- PMCID: PMC10975543
- DOI: 10.3390/molecules29061200
Redox Reactivity of Nonsymbiotic Phytoglobins towards Nitrite
Abstract
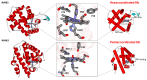
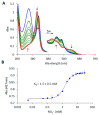
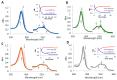
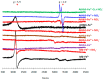
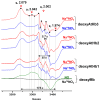
Nonsymbiotic phytoglobins (nsHbs) are a diverse superfamily of hemoproteins grouped into three different classes (1, 2, and 3) based on their sequences. Class 1 Hb are expressed under hypoxia, osmotic stress, and/or nitric oxide exposure, while class 2 Hb are induced by cold stress and cytokinins. Both are mainly six-coordinated. The deoxygenated forms of the class 1 and 2 nsHbs from A. thaliana (AtHb1 and AtHb2) are able to reduce nitrite to nitric oxide via a mechanism analogous to other known globins. NsHbs provide a viable pH-dependent pathway for NO generation during severe hypoxia via nitrite reductase-like activity with higher rate constants compared to mammalian globins. These high kinetic parameters, along with the relatively high concentrations of nitrite present during hypoxia, suggest that plant hemoglobins could indeed serve as anaerobic nitrite reductases in vivo. The third class of nsHb, also known as truncated hemoglobins, have a compact 2/2 structure and are pentacoordinated, and their exact physiological role remains mostly unknown. To date, no reports are available on the nitrite reductase activity of the truncated AtHb3. In the present work, three representative nsHbs of the plant model Arabidopsis thaliana are presented, and their nitrite reductase-like activity and involvement in nitrosative stress is discussed. The reaction kinetics and mechanism of nitrite reduction by nsHbs (deoxy and oxy form) at different pHs were studied by means of UV-Vis spectrophotometry, along with EPR spectroscopy. The reduction of nitrite requires an electron supply, and it is favored in acidic conditions. This reaction is critically affected by molecular oxygen, since oxyAtHb will catalyze nitric oxide deoxygenation. The process displays unique autocatalytic kinetics with metAtHb and nitrate as end-products for AtHb1 and AtHb2 but not for the truncated one, in contrast with mammalian globins.
Keywords: hemoglobin; nitric oxide; nitrite; nitrite oxidase activity; nitrite reductase activity; nonsymbiotic hemoglobin; phytoglobins; redox reactivity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

