Modified NiFe2O4-Supported Graphene Oxide for Effective Urea Electrochemical Oxidation and Water Splitting Applications
- PMID: 38542852
- PMCID: PMC10974038
- DOI: 10.3390/molecules29061215
Modified NiFe2O4-Supported Graphene Oxide for Effective Urea Electrochemical Oxidation and Water Splitting Applications
Abstract
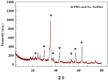
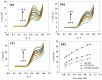
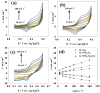
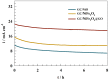
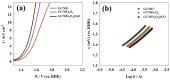
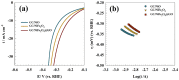
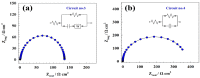
The production of green hydrogen using water electrolysis is widely regarded as one of the most promising technologies. On the other hand, the oxygen evolution reaction (OER) is thermodynamically unfavorable and needs significant overpotential to proceed at a sufficient rate. Here, we outline important structural and chemical factors that affect how well a representative nickel ferrite-modified graphene oxide electrocatalyst performs in efficient water splitting applications. The activities of the modified pristine and graphene oxide-supported nickel ferrite were thoroughly characterized in terms of their structural, morphological, and electrochemical properties. This research shows that the NiFe2O4@GO electrode has an impact on both the urea oxidation reaction (UOR) and water splitting applications. NiFe2O4@GO was observed to have a current density of 26.6 mA cm-2 in 1.0 M urea and 1.0 M KOH at a scan rate of 20 mV s-1. The Tafel slope provided for UOR was 39 mV dec-1, whereas the GC/NiFe2O4@GO electrode reached a current of 10 mA cm-2 at potentials of +1.5 and -0.21 V (vs. RHE) for the OER and hydrogen evolution reaction (HER), respectively. Furthermore, charge transfer resistances were estimated for OER and HER as 133 and 347 Ω cm2, respectively.
Keywords: fuel cells; graphene oxide; nickel ferrite; urea electrooxidation; water splitting.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures






Similar articles
-
NiFe2O4 Nanoparticles/NiFe Layered Double-Hydroxide Nanosheet Heterostructure Array for Efficient Overall Water Splitting at Large Current Densities.ACS Appl Mater Interfaces. 2018 Aug 8;10(31):26283-26292. doi: 10.1021/acsami.8b07835. Epub 2018 Jul 25. ACS Appl Mater Interfaces. 2018. PMID: 30009602
-
Boosting the Production of Hydrogen from an Overall Urea Splitting Reaction Using a Tri-Functional Scandium-Cobalt Electrocatalyst.Small. 2024 Dec;20(49):e2405939. doi: 10.1002/smll.202405939. Epub 2024 Sep 24. Small. 2024. PMID: 39318087
-
Boosting the Performance of Alkaline Anion Exchange Membrane Water Electrolyzer with Vanadium-Doped NiFe2O4.Small. 2025 Feb;21(7):e2410006. doi: 10.1002/smll.202410006. Epub 2025 Jan 7. Small. 2025. PMID: 39777981
-
The polyoxometalates mediated preparation of phosphate-modified NiMoO4-x with abundant O-vacancies for H2 production via urea electrolysis.J Colloid Interface Sci. 2023 Jan;629(Pt A):297-309. doi: 10.1016/j.jcis.2022.08.145. Epub 2022 Aug 27. J Colloid Interface Sci. 2023. PMID: 36081209
-
Recent Advances and Perspectives on Coupled Water Electrolysis for Energy-Saving Hydrogen Production.Adv Sci (Weinh). 2025 Feb;12(7):e2411964. doi: 10.1002/advs.202411964. Epub 2025 Jan 7. Adv Sci (Weinh). 2025. PMID: 39777433 Free PMC article. Review.
Cited by
-
Synthesis of spinel Nickel Ferrite (NiFe2O4)/CNT electrocatalyst for ethylene glycol oxidation in alkaline medium.Heliyon. 2024 Aug 3;10(16):e35791. doi: 10.1016/j.heliyon.2024.e35791. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220931 Free PMC article.
-
Synthesis of NiCo2O4 supported on Chitosan for potential adsorption of copper ions in water samples.Sci Rep. 2025 Apr 24;15(1):14402. doi: 10.1038/s41598-025-96777-y. Sci Rep. 2025. PMID: 40274896 Free PMC article.
-
Synthesis of nickel-sphere coated Ni-Mn layer for efficient electrochemical detection of urea.Sci Rep. 2024 Jun 27;14(1):14818. doi: 10.1038/s41598-024-64707-z. Sci Rep. 2024. PMID: 38937495 Free PMC article.
References
-
- Singh R.K., Rajavelu K., Montag M., Schechter A. Advances in catalytic electrooxidation of urea: A review. Energy Technol. 2021;9:2100017. doi: 10.1002/ente.202100017. - DOI
-
- Sayed E.T., Eisa T., Mohamed H.O., Abdelkareem M.A., Allagui A., Alawadhi H., Chae K.-J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources. 2019;417:159–175. doi: 10.1016/j.jpowsour.2018.12.024. - DOI
-
- Xu W., Wu Z., Tao S. Urea-Based Fuel Cells and Electrocatalysts for Urea Oxidation. Energy Technol. 2016;4:1329–1337. doi: 10.1002/ente.201600185. - DOI
-
- Lan R., Tao S., Irvine J.T.S. A direct urea fuel cell—Power from fertiliser and waste. Energy Environ. Sci. 2010;3:438–441. doi: 10.1039/b924786f. - DOI
-
- Ke K., Wang G., Cao D., Wang G. Electrocatalysis. Springer; Berlin/Heidelberg, Germany: 2020. Recent Advances in the Electro-Oxidation of Urea for Direct Urea Fuel Cell and Urea Electrolysis; pp. 41–78.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

