Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity
- PMID: 38542875
- PMCID: PMC10975738
- DOI: 10.3390/molecules29061238
Biphasic Fermentation of Trapa bispinosa Shells by Ganoderma sinense and Characterization of Its Polysaccharides and Alcoholic Extract and Analysis of Their Bioactivity
Abstract
Background: Trapa bispinosa shells (TBs) and its flesh (TBf) have been recognized for their medicinal properties, including antioxidant, antitumor, and immunomodulatory effects. Despite these benefits, TBs are often discarded as waste material, and their applications remain to be further explored.
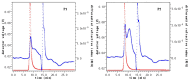
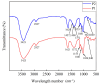
Methods: In this study, we optimized the solid-state fermentation process of Ganoderma sinense (GS) with TBs using a response surface experiment methodology to obtain the fermented production with the highest water extract rate and DPPH free radical scavenging activity. We prepared and characterized pre-fermentation purified polysaccharides (P1) and post-fermentation purified polysaccharides (P2). Alcoholic extracts before (AE1) and after (AE2) fermentation were analyzed for active components such as polyphenols and flavonoids using UPLC-QTOF-MS/MS (ultra-performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry). Mouse macrophages (RAW 264.7) were employed to compare the immune-stimulating ability of polysaccharides and the antioxidant activity of AE1 and AE2.
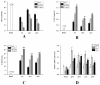
Results: Optimal fermentation conditions comprised a duration of 2 days, a temperature of 14 °C, and a humidity of 77%. The peak water extract yield and DPPH free radical scavenging rate of the water extract from TBs fermented by GS were observed under these conditions. The enhanced activity may be attributed to changes in the polysaccharide structure and the components of the alcoholic extract. The P2 treatment group indicated more secretion of RAW 264.7 cells of NO, iNOS, IL-2, IL-10, and TNF-α than P1, which shows that the polysaccharides demonstrated increased immune-stimulating ability, with their effect linked to the NF-кB pathway. Moreover, the results of the AE2 treatment group indicated that secretion of RAW 264.7 cells of T-AOC and T-SOD increased and MDA decreased, which shows that the alcoholic extract demonstrated enhanced antioxidant activity, with its effect linked to the Nrf2/Keap1-ARE pathway.
Conclusions: Biphasic fermentation of Trapa bispinosa shells by Ganoderma sinense could change the composition and structure of the polysaccharides and the composition of the alcoholic extract, which could increase the products' immunomodulatory and antioxidant activity.
Keywords: Ganoderma sinense; Trapa bispinosa shell; UPLC-QTOF-MS/MS; antioxidant activity; immune activity; polysaccharides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Polysaccharides from Ganoderma Sinense - rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer.BMC Complement Med Ther. 2021 Jun 10;21(1):169. doi: 10.1186/s12906-021-03346-7. BMC Complement Med Ther. 2021. PMID: 34112172 Free PMC article.
-
Optimization of Submerged Fermentation Conditions for Polysaccharide Production in Species of the Genus Ganoderma (Agaricomycetes) and Comparative Analysis of the Antioxidant Activities of Different Strains.Int J Med Mushrooms. 2025;27(1):13-27. doi: 10.1615/IntJMedMushrooms.2024056392. Int J Med Mushrooms. 2025. PMID: 39717915
-
Screening and analysis of potential anti-tumor components from the stipe of Ganoderma sinense using high-performance liquid chromatography/time-of-flight mass spectrometry with multivariate statistical tool.J Chromatogr A. 2017 Mar 3;1487:162-167. doi: 10.1016/j.chroma.2017.01.044. Epub 2017 Jan 22. J Chromatogr A. 2017. PMID: 28143662
-
Ganoderma sinense polysaccharide: An adjunctive drug used for cancer treatment.Prog Mol Biol Transl Sci. 2019;163:165-177. doi: 10.1016/bs.pmbts.2019.02.008. Epub 2019 Mar 25. Prog Mol Biol Transl Sci. 2019. PMID: 31030747 Review.
-
Overview of Ganoderma sinense polysaccharide-an adjunctive drug used during concurrent Chemo/Radiation therapy for cancer treatment in China.Biomed Pharmacother. 2017 Dec;96:865-870. doi: 10.1016/j.biopha.2017.09.060. Epub 2017 Nov 6. Biomed Pharmacother. 2017. PMID: 29078264 Review.
Cited by
-
Facile preparation of fluorescent carbon dots from water caltrop shells and their application in amikacin sensing.RSC Adv. 2025 Mar 11;15(10):7742-7749. doi: 10.1039/d5ra00501a. eCollection 2025 Mar 6. RSC Adv. 2025. PMID: 40070397 Free PMC article.
References
-
- Takeshita S., Ishioka Y., Yagi M., Uemura T., Yamada M., Yonei Y. The effects of water chestnut (Trapa bispinosa Roxb.) on the inhibition of glycometabolism and the improvement in postprandial blood glucose levels in humans. Glycative Stress Res. 2016;3:24–132.
-
- Tania Wahed T.W., Islam M.F., Ratna Sarkar R.S., Sonia Wahed W.S. Phytochemical, antioxidant and cytotoxicity study of the methanolic extracts of leaves of Trapa bispinosa Roxb. Int. J. Pharm. Sci. Res. 2014;10:4400–4405.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

