Nodakenin Ameliorates Ovariectomy-Induced Bone Loss by Regulating Gut Microbiota
- PMID: 38542877
- PMCID: PMC10976110
- DOI: 10.3390/molecules29061240
Nodakenin Ameliorates Ovariectomy-Induced Bone Loss by Regulating Gut Microbiota
Abstract
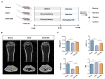
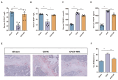
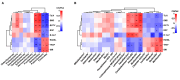
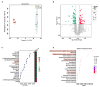
Disordered gut microbiota (GM) structure and function may contribute to osteoporosis (OP). Nodakenin has been shown to ameliorate osteoporosis; however, its anti-osteoporotic mechanism is unknown. This study aimed to further reveal the mechanism of the anti-osteoporotic action of nodakenin from the perspective of the microbiome and metabolome. An osteoporosis model was induced in mice through ovariectomy (OVX), with bone mass and microstructure assessed using μCT. Subsequently, ELISA and histologic examination were used to detect biochemical indicators of bone conversion and intestinal morphology. Using metabolomics and 16S rRNA sequencing, it was possible to determine the composition and abundance of the gut microbiota in feces. The results revealed that nodakenin treatment improved the bone microstructure and serum levels of bone turnover markers, and increased the intestinal mucosal integrity. 16S rRNA sequencing analysis revealed that nodakenin treatment decreased the relative abundance of Firmicutes and Patescibacteria, as well as the F/B ratio, and elevated the relative abundance of Bacteroidetes in OVX mice. In addition, nodakenin enhanced the relative abundance of Muribaculaceae and Allobaculum, among others, at the genus level. Moreover, metabolomics analysis revealed that nodakenin treatment significantly altered the changes in 113 metabolites, including calcitriol. A correlation analysis revealed substantial associations between various gut microbiota taxa and both the osteoporosis phenotype and metabolites. In summary, nodakenin treatment alleviated OVX-induced osteoporosis by modulating the gut microbiota and intestinal barrier.
Keywords: gut microbiota; intestinal barrier; metabolomics; nodakenin; osteoporosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Irisin mitigates osteoporotic-associated bone loss and gut dysbiosis in ovariectomized mice by modulating microbiota, metabolites, and intestinal barrier integrity.BMC Musculoskelet Disord. 2025 Apr 16;26(1):374. doi: 10.1186/s12891-025-08622-y. BMC Musculoskelet Disord. 2025. PMID: 40241040 Free PMC article.
-
Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function.J Orthop Translat. 2022 Sep 26;37:46-60. doi: 10.1016/j.jot.2022.08.003. eCollection 2022 Nov. J Orthop Translat. 2022. PMID: 36196151 Free PMC article.
-
An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice.Am J Clin Nutr. 2021 Oct 4;114(4):1304-1313. doi: 10.1093/ajcn/nqab194. Am J Clin Nutr. 2021. PMID: 34113963
-
Changes in the gut microbiota of osteoporosis patients based on 16S rRNA gene sequencing: a systematic review and meta-analysis.J Zhejiang Univ Sci B. 2022 Dec 15;23(12):1002-1013. doi: 10.1631/jzus.B2200344. J Zhejiang Univ Sci B. 2022. PMID: 36518053 Free PMC article.
-
Targeting the gut microbiota-related metabolites for osteoporosis: The inextricable connection of gut-bone axis.Ageing Res Rev. 2024 Feb;94:102196. doi: 10.1016/j.arr.2024.102196. Epub 2024 Jan 12. Ageing Res Rev. 2024. PMID: 38218463 Review.
Cited by
-
From gut to bone: deciphering the impact of gut microbiota on osteoporosis pathogenesis and management.Front Cell Infect Microbiol. 2024 Sep 25;14:1416739. doi: 10.3389/fcimb.2024.1416739. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39386168 Free PMC article. Review.
-
Case Report: Therapeutic potential of traditional Korean herbal medicine Jeopgol-tang in bone regeneration: a case series of delayed union over 5 months.Front Endocrinol (Lausanne). 2025 Jun 30;16:1595784. doi: 10.3389/fendo.2025.1595784. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40661740 Free PMC article.
References
-
- Ishikawa K., Nagai T., Sakamoto K., Ohara K., Eguro T., Ito H., Toyoshima Y., Kokaze A., Toyone T., Inagaki K. High bone turnover elevates the risk of denosumab-induced hypocalcemia in women with postmenopausal osteoporosis. Ther. Clin. Risk Manag. 2016;12:1831–1840. doi: 10.2147/TCRM.S123172. - DOI - PMC - PubMed
-
- Zhang Y.W., Cao M.M., Li Y.J., Dai G.C., Lu P.P., Zhang M., Bai L.Y., Chen X.X., Shi L., Zhang C., et al. Dietary Protein Intake in Relation to the Risk of Osteoporosis in Middle-Aged and Older Individuals: A Cross-Sectional Study. J. Nutr. Health Aging. 2022;26:252–258. doi: 10.1007/s12603-022-1748-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

