State-to-State Quantum Dynamics Study of Intramolecular Isotope Effects on Be(1S) + HD (v0 = 2, j0 = 0) → BeH/BeD + H/D Reaction
- PMID: 38542900
- PMCID: PMC10975831
- DOI: 10.3390/molecules29061263
State-to-State Quantum Dynamics Study of Intramolecular Isotope Effects on Be(1S) + HD (v0 = 2, j0 = 0) → BeH/BeD + H/D Reaction
Abstract
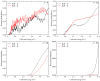
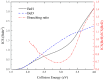
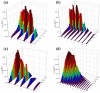
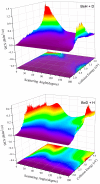
The dynamic mechanisms and intramolecular isotope effects of the Be(1S) + HD (v0 = 2, j0 = 0) → BeH/BeD + H/D reaction are studied at the state-to-state level using the time-dependent wave packet method on a high-quality potential energy surface. This reaction can proceed along the indirect pathway that features a barrier and a deep well or the smooth direct pathway. The reaction probabilities, total and state-resolved integral cross sections, and differential cross sections are analyzed in detail. The calculated dynamics results show that both of the products are mainly formed by the dissociation of a collinear HBeD intermediate when the collision energy is slightly larger than the threshold. As the collision energy increases, the BeH + D channel is dominated by the direct abstraction process, whereas the BeD + H channel mainly follows the complex-forming mechanism.
Keywords: integral cross section; intramolecular isotope effect; quantum dynamics; time-dependent wave packet.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
A Globally Accurate Neural Network Potential Energy Surface and Quantum Dynamics Studies on Be+(2S) + H2/D2 → BeH+/BeD+ + H/D Reactions.Molecules. 2024 Jul 22;29(14):3436. doi: 10.3390/molecules29143436. Molecules. 2024. PMID: 39065017 Free PMC article.
-
Quantum Dynamics Studies of the Significant Intramolecular Isotope Effects on the Nonadiabatic Be+(2P) + HD → BeH+/BeD+ + D/H Reaction.J Phys Chem A. 2021 Jan 14;125(1):235-242. doi: 10.1021/acs.jpca.0c09593. Epub 2020 Dec 28. J Phys Chem A. 2021. PMID: 33369408
-
Ab Initio Neural Network Potential Energy Surface and Quantum Dynamics Calculations on Na(2S) + H2 → NaH + H Reaction.Molecules. 2024 Oct 14;29(20):4871. doi: 10.3390/molecules29204871. Molecules. 2024. PMID: 39459240 Free PMC article.
-
A time-dependent wave packet quantum scattering study of the reaction HD+ (v = 0 - 3;j0 = 1) + He --> HeH+(HeD+) + D(H).J Chem Phys. 2007 Oct 28;127(16):164318. doi: 10.1063/1.2800009. J Chem Phys. 2007. PMID: 17979349
-
Quantum dynamics studies of isotope effects in the Mg+(3p) + HD → MgH+/MgD+ + D/H insertion reaction.Sci Rep. 2020 Feb 25;10(1):3410. doi: 10.1038/s41598-020-60033-2. Sci Rep. 2020. PMID: 32098984 Free PMC article.
Cited by
-
A Globally Accurate Neural Network Potential Energy Surface and Quantum Dynamics Studies on Be+(2S) + H2/D2 → BeH+/BeD+ + H/D Reactions.Molecules. 2024 Jul 22;29(14):3436. doi: 10.3390/molecules29143436. Molecules. 2024. PMID: 39065017 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

