Experimental and Computational Methods to Assess Central Nervous System Penetration of Small Molecules
- PMID: 38542901
- PMCID: PMC10975190
- DOI: 10.3390/molecules29061264
Experimental and Computational Methods to Assess Central Nervous System Penetration of Small Molecules
Abstract
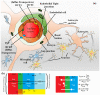
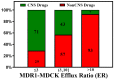
In CNS drug discovery, the estimation of brain exposure to lead compounds is critical for their optimization. Compounds need to cross the blood-brain barrier (BBB) to reach the pharmacological targets in the CNS. The BBB is a complex system involving passive and active mechanisms of transport and efflux transporters such as P-glycoproteins (P-gp) and breast cancer resistance protein (BCRP), which play an essential role in CNS penetration of small molecules. Several in vivo, in vitro, and in silico methods are available to estimate human brain penetration. Preclinical species are used as in vivo models to understand unbound brain exposure by deriving the Kp,uu parameter and the brain/plasma ratio of exposure corrected with the plasma and brain free fraction. The MDCK-mdr1 (Madin Darby canine kidney cells transfected with the MDR1 gene encoding for the human P-gp) assay is the commonly used in vitro assay to estimate compound permeability and human efflux. The in silico methods to predict brain exposure, such as CNS MPO, CNS BBB scores, and various machine learning models, help save costs and speed up compound discovery and optimization at all stages. These methods enable the screening of virtual compounds, building of a CNS penetrable compounds library, and optimization of lead molecules for CNS penetration. Therefore, it is crucial to understand the reliability and ability of these methods to predict CNS penetration. We review the in silico, in vitro, and in vivo data and their correlation with each other, as well as assess published experimental and computational approaches to predict the BBB penetrability of compounds.
Keywords: CNS drug discovery; P-glycoproteins (P-gp); active transport; blood–brain barrier (BBB); breast cancer resistance protein (BCRP); efflux transporters; in silico models; influx transporters; passive diffusion.
Conflict of interest statement
Mayuri Gupta is employed by Merck & Co., Inc.
Figures

Similar articles
-
Characterization and Validation of Canine P-Glycoprotein-Deficient MDCK II Cell Lines for Efflux Substrate Screening.Pharm Res. 2020 Sep 11;37(10):194. doi: 10.1007/s11095-020-02895-9. Pharm Res. 2020. PMID: 32918191
-
Harnessing Preclinical Data as a Predictive Tool for Human Brain Tissue Targeting.ACS Chem Neurosci. 2021 Mar 17;12(6):1007-1017. doi: 10.1021/acschemneuro.0c00807. Epub 2021 Mar 2. ACS Chem Neurosci. 2021. PMID: 33651587
-
Utilizing a Dual Human Transporter MDCKII-MDR1-BCRP Cell Line to Assess Efflux at the Blood Brain Barrier.Drug Metab Dispos. 2024 Jan 9;52(2):95-105. doi: 10.1124/dmd.123.001476. Drug Metab Dispos. 2024. PMID: 38071533
-
Recent advances in the in vitro and in vivo methods to assess impact of P-glycoprotein and breast cancer resistance protein transporters in central nervous system drug disposition.Biopharm Drug Dispos. 2023 Feb;44(1):7-25. doi: 10.1002/bdd.2345. Epub 2023 Feb 5. Biopharm Drug Dispos. 2023. PMID: 36692150 Review.
-
A Practical Perspective on the Evaluation of Small Molecule CNS Penetration in Drug Discovery.Drug Metab Lett. 2019;13(2):78-94. doi: 10.2174/1872312813666190311125652. Drug Metab Lett. 2019. PMID: 30854983 Review.
Cited by
-
Applicability of MDR1 Overexpressing Abcb1KO-MDCKII Cell Lines for Investigating In Vitro Species Differences and Brain Penetration Prediction.Pharmaceutics. 2024 May 29;16(6):736. doi: 10.3390/pharmaceutics16060736. Pharmaceutics. 2024. PMID: 38931858 Free PMC article.
-
The current approaches to modeling the brain ischemia-reperfusion and inflammation: from animal models toward vascularized and neuroimmune cerebral organoids.Rev Neurosci. 2025 May 28. doi: 10.1515/revneuro-2025-0015. Online ahead of print. Rev Neurosci. 2025. PMID: 40440504 Review.
-
Prodrug Approach as a Strategy to Enhance Drug Permeability.Pharmaceuticals (Basel). 2025 Feb 21;18(3):297. doi: 10.3390/ph18030297. Pharmaceuticals (Basel). 2025. PMID: 40143076 Free PMC article. Review.
-
Systematic Study of Steroid Drugs' Ability to Cross Biomembranes-The Possible Environmental Impact and Health Risks Associated with Exposure During Pregnancy.Membranes (Basel). 2024 Dec 26;15(1):4. doi: 10.3390/membranes15010004. Membranes (Basel). 2024. PMID: 39852245 Free PMC article.
-
Log BB Prediction Models Using TLC and HPLC Retention Values as Protein Affinity Data.Pharmaceutics. 2024 Nov 30;16(12):1534. doi: 10.3390/pharmaceutics16121534. Pharmaceutics. 2024. PMID: 39771513 Free PMC article.
References
-
- Stephens R.H., O’Neill C.A., Bennett J., Humphrey M., Henry B., Rowland M., Warhurst G. Resolution of P-glycoprotein and non-P-glycoprotein effects on drug permeability using intestinal tissues from mdr1a (−/−) mice. Br. J. Pharmacol. 2002;135:2038–2046. doi: 10.1038/sj.bjp.0704668. - DOI - PMC - PubMed
-
- Dickens D., Radisch S., Pirmohamed M. Drug Transporters: Volume 1: Role and Importance in ADME and Drug Development. Volume 1. The Royal Society of Chemistry; London, UK: 2016. Chapter 5 Drug Transporters at the Blood–Brain Barrier; pp. 151–183.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

