Oral Delivery of Astaxanthin via Carboxymethyl Chitosan-Modified Nanoparticles for Ulcerative Colitis Treatment
- PMID: 38542929
- PMCID: PMC10975021
- DOI: 10.3390/molecules29061291
Oral Delivery of Astaxanthin via Carboxymethyl Chitosan-Modified Nanoparticles for Ulcerative Colitis Treatment
Abstract
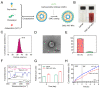
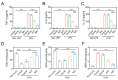
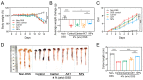
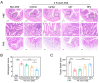
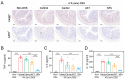
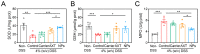
The oral delivery strategy of natural anti-oxidant and anti-inflammatory agents has attracted great attention to improve the effectiveness of ulcerative colitis (UC) treatment. Herein, we developed a novel orally deliverable nanoparticle, carboxymethyl chitosan (CMC)-modified astaxanthin (AXT)-loaded nanoparticles (CMC-AXT-NPs), for UC treatment. The CMC-AXT-NPs were evaluated by appearance, morphology, particle size, ζ-potential, and encapsulation efficiency (EE). The results showed that CMC-AXT-NPs were nearly spherical in shape with a particle size of 34.5 nm and ζ-potential of -30.8 mV, and the EE of CMC-AXT-NPs was as high as 95.03%. The CMC-AXT-NPs exhibited preferable storage stability over time and well-controlled drug-release properties in simulated intestinal fluid. Additionally, in vitro studies revealed that CMC-AXT-NPs remarkably inhibited cytotoxicity induced by LPS and demonstrated superior antioxidant and anti-inflammatory abilities in Raw264.7 cells. Furthermore, CMC-AXT-NPs effectively alleviated clinical symptoms of colitis induced by dextran sulfate sodium salt (DSS), including maintaining body weight, inhibiting colon shortening, and reducing fecal bleeding. Importantly, CMC-AXT-NPs suppressed the expression of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β and ameliorated DSS-induced oxidative damage. Our results demonstrated the potential of CMC-modified nanoparticles as an oral delivery system and suggested these novel AXT nanoparticles could be a promising strategy for UC treatment.
Keywords: anti-inflammatory; astaxanthin; carboxymethyl chitosan; nanoparticle; ulcerative colitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

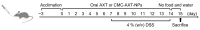
Similar articles
-
An efficient co-delivery system based on multilayer structural nanoparticles for programmed sequential release of resveratrol and vitamin D3 to combat dextran sodium sulfate-induced colitis in mice.Int J Biol Macromol. 2024 Jan;254(Pt 3):127962. doi: 10.1016/j.ijbiomac.2023.127962. Epub 2023 Nov 10. Int J Biol Macromol. 2024. PMID: 37952331
-
Cyclosporine A-loaded colon-targeted oral nanomicelles self-assembly by galactosylated carboxymethyl chitosan for efficient ulcerative colitis therapy.Eur J Pharm Biopharm. 2023 Aug;189:152-164. doi: 10.1016/j.ejpb.2023.06.010. Epub 2023 Jun 17. Eur J Pharm Biopharm. 2023. PMID: 37336365
-
Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis.J Crohns Colitis. 2018 Jan 24;12(2):217-229. doi: 10.1093/ecco-jcc/jjx115. J Crohns Colitis. 2018. PMID: 28961808 Free PMC article.
-
Astaxanthin biosynthesis for functional food development and space missions.Crit Rev Biotechnol. 2025 Jun;45(4):923-937. doi: 10.1080/07388551.2024.2410364. Epub 2024 Oct 20. Crit Rev Biotechnol. 2025. PMID: 39428346 Review.
-
Astaxanthin in cancer therapy and prevention (Review).Biomed Rep. 2025 Feb 14;22(4):66. doi: 10.3892/br.2025.1944. eCollection 2025 Apr. Biomed Rep. 2025. PMID: 40017498 Free PMC article. Review.
Cited by
-
Medical Applications and Cellular Mechanisms of Action of Carboxymethyl Chitosan Hydrogels.Molecules. 2024 Sep 13;29(18):4360. doi: 10.3390/molecules29184360. Molecules. 2024. PMID: 39339355 Free PMC article. Review.
-
Protective Effects of Astaxanthin against Oxidative Stress: Attenuation of TNF-α-Induced Oxidative Damage in SW480 Cells and Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in C57BL/6 Mice.Mar Drugs. 2024 Oct 12;22(10):469. doi: 10.3390/md22100469. Mar Drugs. 2024. PMID: 39452878 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

