Advances and Prospects in the Study of Spherical Polyelectrolyte Brushes as a Dopant for Conducting Polymers
- PMID: 38542950
- PMCID: PMC10976150
- DOI: 10.3390/molecules29061315
Advances and Prospects in the Study of Spherical Polyelectrolyte Brushes as a Dopant for Conducting Polymers
Abstract
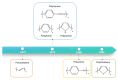
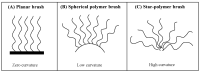
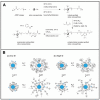
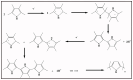
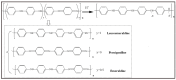
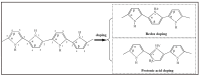
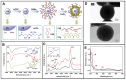
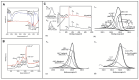
Owing to their special structure and excellent physical and chemical properties, conducting polymers have attracted increasing attention in materials science. In recent years, tremendous efforts have been devoted to improving the comprehensive performance of conducting polymers by using the technique of "doping." Spherical polyelectrolyte brushes (SPBs) bearing polyelectrolyte chains grafted densely to the surface of core particles have the potential to be novel dopant of conducting polymers not only because of their spherical structure, high grafting density and high charge density, but also due to the possibility of their being applied in printed electronics. This review first presents a summary of the general dopants of conducting polymers. Meanwhile, conducting polymers doped with spherical polyelectrolyte brushes (SPBs) is highlighted, including the preparation, characterization, performance and doping mechanism. It is demonstrated that comprehensive performance of conducting polymers has improved with the addition of SPBs, which act as template and dopant in the synthesis of composites. Furthermore, the applications and future developments of conductive composites are also briefly reviewed and proposed, which would draw more attention to this field.
Keywords: conducting polymers; dopants; doping mechanism; spherical polyelectrolyte brushes.
Conflict of interest statement
The author declares no conflict of interest.
Figures











Similar articles
-
Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking.Molecules. 2023 Dec 7;28(24):7984. doi: 10.3390/molecules28247984. Molecules. 2023. PMID: 38138474 Free PMC article. Review.
-
Electrophoresis and dielectric dispersion of spherical polyelectrolyte brushes.Langmuir. 2012 Nov 27;28(47):16372-81. doi: 10.1021/la302483e. Epub 2012 Nov 13. Langmuir. 2012. PMID: 23110617
-
Improving Electrical Conductivity, Thermal Stability, and Solubility of Polyaniline-Polypyrrole Nanocomposite by Doping with Anionic Spherical Polyelectrolyte Brushes.Nanoscale Res Lett. 2015 Dec;10(1):997. doi: 10.1186/s11671-015-0997-x. Epub 2015 Jul 25. Nanoscale Res Lett. 2015. PMID: 26209298 Free PMC article.
-
Achieving Efficient n-Doping of Conjugated Polymers by Molecular Dopants.Acc Chem Res. 2021 Jul 6;54(13):2871-2883. doi: 10.1021/acs.accounts.1c00223. Epub 2021 Jun 21. Acc Chem Res. 2021. PMID: 34152131
-
Interaction of proteins with linear polyelectrolytes and spherical polyelectrolyte brushes in aqueous solution.Phys Chem Chem Phys. 2006 Dec 7;8(45):5269-75. doi: 10.1039/b609879g. Phys Chem Chem Phys. 2006. PMID: 19810405 Review.
Cited by
-
Dipolar Brush Polymers: A Numerical Study of the Force Exerted onto a Penetrating Colloidal Particle Under an External Field.Polymers (Basel). 2025 Jan 29;17(3):366. doi: 10.3390/polym17030366. Polymers (Basel). 2025. PMID: 39940567 Free PMC article.
-
Recent Progress in Flexible Wearable Sensors Utilizing Conductive Hydrogels for Sports Applications: Characteristics, Mechanisms, and Modification Strategies.Gels. 2025 Jul 30;11(8):589. doi: 10.3390/gels11080589. Gels. 2025. PMID: 40868720 Free PMC article. Review.
References
-
- Shirakawa H., Louis E.L., MacDiarmid A.G. Synthesis of electrically conducting organic polymers: Halogen derivatives of Polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977;16:578–580. doi: 10.1039/c39770000578. - DOI
-
- Tissera N.D., Wijesena R.N., Rathnayake S., Silva R.M., Nalin de Silva K.M. Heterogeneous in situ polymerization of polyaniline (PANI) nanofibers on cotton textiles: Improved electrical conductivity, electrical switching, and tuning properties. Carbohydrate polymers. Carbohyd. Polym. 2018;186:35–44. doi: 10.1016/j.carbpol.2018.01.027. - DOI - PubMed
-
- Hien H.T., Tuan C.V., Thu D.T.A., Ngan P.Q., Thai G.H., Doanh S.C., Giang H.T., Van N.D., Trung T. Influence of surface morphology and doping of PPy film simultaneously polymerized by vapour phase oxidation on gas sensing. Synth. Met. 2019;250:35–41. doi: 10.1016/j.synthmet.2019.02.013. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

