Synthetic Methods and Pharmacological Potentials of Triazolothiadiazines: A Review
- PMID: 38542962
- PMCID: PMC10975507
- DOI: 10.3390/molecules29061326
Synthetic Methods and Pharmacological Potentials of Triazolothiadiazines: A Review
Abstract
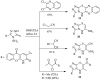
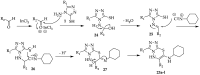
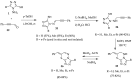
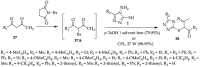
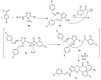
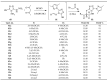
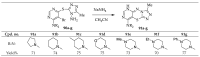
This review article examines the synthetic pathways for triazolothiadiazine derivatives, such as triazolo[3,4-b]thiadiazines, triazolo[5,1-b]thiadiazines, and triazolo[4,3-c]thiadiazines, originating from triazole derivatives, thiadiazine derivatives, or thiocarbohydrazide. The triazolothiadiazine derivatives exhibit several biological actions, including antibacterial, anticancer, antiviral, antiproliferative, analgesic, anti-inflammatory, and antioxidant properties. The review article aims to assist researchers in creating new biologically active compounds for designing target-oriented triazolothiadiazine-based medicines to treat multifunctional disorders.
Keywords: biological activity; thiadiazine; thiocarbohydrazide; triazolo[3,4-b]thiadiazines; triazolo[4,3-c]thiadiazines triazol; triazolo[5,1-b]thiadiazines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




















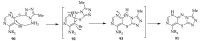
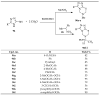
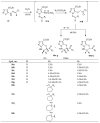
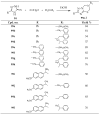


Similar articles
-
Vision on Synthetic and Medicinal Facets of 1,2,4-Triazolo[3,4-b][1,3,4]thiadiazine Scaffold.Top Curr Chem (Cham). 2022 Feb 5;380(2):10. doi: 10.1007/s41061-022-00365-x. Top Curr Chem (Cham). 2022. PMID: 35122161 Free PMC article. Review.
-
Design and Synthesis of 1,2,4-Triazolo[3,2-b]-1,3,5-thiadiazine Derivatives as a Novel Template for Analgesic/Anti-Inflammatory Activity.Arch Pharm (Weinheim). 2017 Jul;350(7). doi: 10.1002/ardp.201700052. Epub 2017 Jun 8. Arch Pharm (Weinheim). 2017. PMID: 28594090
-
New triazole and triazolothiadiazine derivatives as possible antimicrobial agents.Eur J Med Chem. 2008 Jan;43(1):155-9. doi: 10.1016/j.ejmech.2007.03.019. Epub 2007 Apr 5. Eur J Med Chem. 2008. PMID: 17499887
-
Studies on arylfuran derivatives--Part V. Synthesis and antibacterial properties of 7H,6-[5-(4-nitrophenyl)-2-furyl]-1,2,4-triazolo [3,4-b]-1,3,4-thiadiazines.Boll Chim Farm. 1996 Jul-Aug;135(7):447-51. Boll Chim Farm. 1996. PMID: 9035556
-
Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents.J Inorg Biochem. 2005 Aug;99(8):1558-72. doi: 10.1016/j.jinorgbio.2005.05.004. J Inorg Biochem. 2005. PMID: 16005979 Review.
References
-
- Bräse S. Privileged Scaffolds in Medicinal Chemistry. Design, Synthesis, Evaluation. RSC; Cambridge, UK: 2016. - DOI
-
- Xu Q., Bao K., Sun M., Xu J., Wang Y., Tian H., Zuo D., Guan Q., Wu Y., Zhang W. Design, synthesis and structure activity relationship of 3,6-diaryl-7H-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazines as novel tubulin inhibitors. Sci. Rep. 2017;7:11997. doi: 10.1038/s41598-017-10860-7. - DOI - PMC - PubMed
-
- Tolan H., Fahim A., Ismael E. Synthesis, biological activities, molecular docking, theoretical calculations of some 1,3,4-oxadiazoles, 1,2,4-triazoles, and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines derivatives. J. Mole. Struct. 2023;1283:135238. doi: 10.1016/j.molstruc.2023.135238. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

