Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides
- PMID: 38542963
- PMCID: PMC10976143
- DOI: 10.3390/molecules29061327
Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides
Abstract
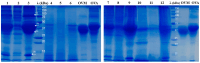
Pepsin, trypsin and proteinase K were used in the present study to hydrolyse the proteins from whole eggs, yolks or whites, and the resulting hydrolysates were characterised in terms of antioxidant and IgE-binding properties, using a combination of in vitro and in silico methods. Based on the degree of hydrolysis (DH) results, the egg yolk proteins are better substrates for all the tested enzymes (DH of 6.2-20.1%) compared to those from egg whites (DH of 2.0-4.4%). The SDS-PAGE analysis indicated that pepsin and proteinase K were more efficient compared to trypsin in breaking the intramolecular peptide bonds of the high molecular weight egg proteins. For all the tested substrates, enzyme-assisted hydrolysis resulted in a significant increase in antioxidant activity, suggesting that many bioactive peptides are encrypted in inactive forms in the parent proteins. The hydrolysates obtained with proteinase K exhibited the highest DPPH radical scavenging activity (124-311 µM Trolox/g protein) and the lowest residual IgE-binding capacity. The bioinformatics tools revealed that proteinase K is able to break the integrity of the main linear IgE-binding epitopes from ovalbumin and ovomucoid. It can be concluded that proteinase K is a promising tool for modulating the intrinsic properties of egg proteins.
Keywords: antigenic properties; antioxidant activity; egg proteins; hydrolysis degree; proteolytic enzymes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- ANSES-CIQUAL Food Composition Table. 2020. [(accessed on 29 January 2024)]. Available online: https://www.anses.fr/en/content/anses-ciqual-food-composition-table.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

