In Vitro and Ex Vivo Antifungal Activities of Metconazole against the Rice Blast Fungus Pyricularia oryzae
- PMID: 38542989
- PMCID: PMC10975861
- DOI: 10.3390/molecules29061353
In Vitro and Ex Vivo Antifungal Activities of Metconazole against the Rice Blast Fungus Pyricularia oryzae
Abstract
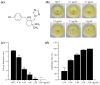
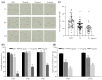
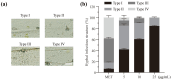
Rice blast, caused by the filamentous fungus Pyricularia oryzae, has long been one of the major threats to almost all rice-growing areas worldwide. Metconazole, 5-(4-chlorobenzyl)-2, 2-dimethyl-1-(1H-1, 2, 4-triazol-1-ylmethyl) cyclopentanol, is a lipophilic, highly active triazole fungicide that has been applied in the control of various fungal pathogens of crops (cereals, barley, wheat), such as the Fusarium and Alternaria species. However, the antifungal activity of metconazole against P. oryzae is unknown. In this study, metconazole exhibited broad spectrum antifungal activities against seven P. oryzae strains collected from rice paddy fields and the wild type strain P131. Scanning electron microscopic analysis and fluorescein diacetate staining assays revealed that metconazole treatment damaged the cell wall integrity, cell membrane permeability and even cell viability of P. oryzae, resulting in deformed and shrunken hyphae. The supplementation of metconazole in vitro increased fungal sensitivity to different stresses, such as sodium dodecyl sulfate, congo red, sodium chloride, sorbitol and oxidative stress (H2O2). Metconazole could inhibit key virulence processes of P. oryzae, including conidial germination, germ tube elongation and appressorium formation. Furthermore, this chemical prevented P. oryzae from infecting barley epidermal cells by disturbing appressorium penetration and subsequent invasive hyphae development. Pathogenicity assays indicated a reduction of over 75% in the length of blast lesions in both barley and rice leaves when 10 μg/mL of metconazole was applied. This study provides evidence to understand the antifungal effects of metconazole against P. oryzae and demonstrates its potential in rice blast management.
Keywords: Pyricularia oryzae; antifungal activity; fungicide; metconazole; rice blast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A Histone Deacetylase, Magnaporthe oryzae RPD3, Regulates Reproduction and Pathogenic Development in the Rice Blast Fungus.mBio. 2021 Dec 21;12(6):e0260021. doi: 10.1128/mBio.02600-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781734 Free PMC article.
-
Role of Two Metacaspases in Development and Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae.mBio. 2021 Feb 9;12(1):e03471-20. doi: 10.1128/mBio.03471-20. mBio. 2021. PMID: 33563831 Free PMC article.
-
The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide.PLoS One. 2021 Aug 27;16(8):e0256807. doi: 10.1371/journal.pone.0256807. eCollection 2021. PLoS One. 2021. PMID: 34449822 Free PMC article.
-
The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae.Environ Microbiol. 2017 Mar;19(3):1008-1016. doi: 10.1111/1462-2920.13688. Epub 2017 Mar 1. Environ Microbiol. 2017. PMID: 28165657 Review.
-
Pyricularia oryzae: Lab star and field scourge.Mol Plant Pathol. 2024 Apr;25(4):e13449. doi: 10.1111/mpp.13449. Mol Plant Pathol. 2024. PMID: 38619508 Free PMC article. Review.
Cited by
-
Crystal structure and Hirshfeld surface analysis of the fungicide metconazole.Acta Crystallogr E Crystallogr Commun. 2025 Apr 8;81(Pt 5):385-388. doi: 10.1107/S205698902500310X. eCollection 2025 May 1. Acta Crystallogr E Crystallogr Commun. 2025. PMID: 40336890 Free PMC article.
References
-
- Li Y., Wu C., Jiang G., Wang L., He Y. Dynamic analyses of rice blast resistance for the assessment of genetic and environmental effects. Plant Breed. 2007;126:541–547. doi: 10.1111/j.1439-0523.2007.01409.x. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

