Detection of Adulterated Naodesheng Tablet (Naodesheng Pian) via In-Depth Chemical Analysis and Subsequent Reconstruction of Its Pharmacopoeia Q-Markers
- PMID: 38543029
- PMCID: PMC10974483
- DOI: 10.3390/molecules29061392
Detection of Adulterated Naodesheng Tablet (Naodesheng Pian) via In-Depth Chemical Analysis and Subsequent Reconstruction of Its Pharmacopoeia Q-Markers
Abstract
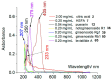
Naodesheng Tablet (Naodesheng Pian), a traditional Chinese medicine formula for stroke treatment, is made up of five herbal medicines, i.e., Sanqi, Gegen, Honghua, Shanzha, and Chuanxiong. However, the current Pharmacopoeia quality-marker (Q-marker) system cannot detect possible adulteration. Our study tried to use a new strategy, i.e., standards-library-dependent ultra-high-performance liquid chromatography-quadrupole-Orbitrap mass spectrometry (UHPLC-Q-Orbitrap MS/MS) putative identification, to reconstruct the Q-marker system. Through the strategy, 30 isomers were successfully differentiated (such as 2'-hydroxygenistein, luteolin, and kaempferol; ginsenoside Rg2 and ginsenoside Rg3; ginsenoside Rf and ginsenoside Rg1). In particular, 11 compounds were unexpectedly found in Naodesheng, including 2'-hydroxygenistein, 7,4'-dihydroxyflavone, pectolinarigenin, 7-methoxy-4'-hydroxyisoflavone, scoparone, matrine, 3,3',4',5,6,7,8-heptamethoxyflavone, 5-hydroxyflavone, diosgenin, chloesteryl acetate, and (+)-4-cholesten-3-one. In total, 68 compounds were putatively identified and fully elucidated for their MS spectra. Subsequently, relevant compounds were further investigated using UV-vis scanning experiments, semi-quantitative analysis, and quantum chemical calculation. Finally, five adulterated Naodesheng Tablets were used for validation experiments. The experiment successfully detected five adulterated ones via a lower-version LC-MS analysis. On this basis, three new candidates (hydroxy safflor yellow A (HSYA), citric acid, and levistilide A), along with puerarin and notoginsenoside R1, are re-nominated as the Q-markers for LC-MS analysis. The LC-MS analysis of puerarin, notoginsenoside R1, HSYA, citric acid, and levistilide A can clearly detect adulteration regarding all five herbal medicines mentioned above. Therefore, the reconstructed Q-markers are described as a "perfect" quality control system to detect adulteration in Naodesheng and will offer a valuable recommendation for the Pharmacopoeia Commission.
Keywords: Naodesheng Pian; UHPLC-Q-Exactive-Orbitrap MS/MS; counterfeiting recognition; quality control.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chinese-Pharmacopoeia-Commission . Chinese Pharmacopoeia (Part 1) Volume 1 Chinese Medical Science and Technology Press; Beijing, China: 2020.
-
- National Medical Products Administration of China Data Search from National Medical Products Administration of China. [(accessed on 2 November 2023)]; Available online: https://www.nmpa.gov.cn/datasearch/
-
- Martinelli F., Perrone A., Yousefi S., Papini A., Castiglione S., Guarino F., Cicatelli A., Aelaei M., Arad N., Gholami M., et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegusmonogyna Jacq.), Rosaceae. Molecules. 2021;26:7266. doi: 10.3390/molecules26237266. - DOI - PMC - PubMed
-
- Wang G., Li Q., Song T., Fan W., Zhang M., Liu Y. Determination of Puerarin and hydroxy safflor yellow A in Nuodesheng soft capsule by HPLC. China Pharm. 2009;12:699–702. (In Chinese)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

