Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B
- PMID: 38543033
- PMCID: PMC10974182
- DOI: 10.3390/molecules29061396
Characterisation of Modular Polyketide Synthases Designed to Make Pentaene Analogues of Amphotericin B
Abstract
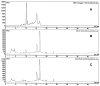
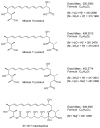
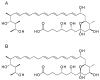
Glycosylated polyene macrolides are important antifungal agents that are produced by many actinomycete species. Development of new polyenes may deliver improved antibiotics. Here, Streptomyces nodosus was genetically re-programmed to synthesise pentaene analogues of the heptaene amphotericin B. These pentaenes are of interest as surrogate substrates for enzymes catalysing unusual, late-stage biosynthetic modifications. The previous deletion of amphotericin polyketide synthase modules 5 and 6 generated S. nodosus M57, which produces an inactive pentaene. Here, the chain-terminating thioesterase was fused to module 16 to generate strain M57-16TE, in which cycles 5, 6, 17 and 18 are eliminated from the biosynthetic pathway. Another variant of M57 was obtained by replacing modules 15, 16 and 17 with a single 15-17 hybrid module. This gave strain M57-1517, in which cycles 5, 6, 15 and 16 are deleted. M57-16TE and M57-1517 gave reduced pentaene yields. Only M57-1517 delivered its predicted full-length pentaene macrolactone in low amounts. For both mutants, the major pentaenes were intermediates released from modules 10, 11 and 12. Longer pentaene chains were unstable. The novel pentaenes were not glycosylated and were not active against Candida albicans. However, random mutagenesis and screening may yet deliver new antifungal producers from the M57-16TE and M57-1517 strains.
Keywords: amphotericin B; antifungal antibiotics; glycosylated polyene macrolides; modular polyketide synthase; synthetic biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

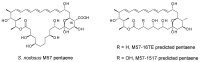



References
-
- Bruheim P., Borgos S.E., Tsan P., Sletta H., Ellingsen T.E., Lancelin J.-M., Zotchev S.B., Stocker H., Kruse G., Kreckel P., et al. Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob. Agents Chemother. 2004;48:4148–4153. doi: 10.1128/AAC.48.11.4120-4129.2004. - DOI - PMC - PubMed
-
- Hogan M., Song Y., Muldoon J., Caffrey P. Generation of new glycoanalogues of polyene antibiotics by synthetic biology—Testing current technical boundaries. SynBio. 2024;2:31–55. doi: 10.3390/synbio2010003. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

