Evolution of the Quinoline Scaffold for the Treatment of Leishmaniasis: A Structural Perspective
- PMID: 38543071
- PMCID: PMC10975785
- DOI: 10.3390/ph17030285
Evolution of the Quinoline Scaffold for the Treatment of Leishmaniasis: A Structural Perspective
Abstract
Since the beginning of the XXI century, Leishmaniasis has been integrated into the World Health Organization's list of the 20 neglected tropical diseases, being considered a public health issue in more than 88 countries, especially in the tropics, subtropics, and the Mediterranean area. Statistically, this disease presents a world prevalence of 12 million cases worldwide, with this number being expected to increase shortly due to the 350 million people considered at risk and the 2-2.5 million new cases appearing every year. The lack of an appropriate and effective treatment against this disease has intensified the interest of many research groups to pursue the discovery and development of novel treatments in close collaboration with the WHO, which hopes to eradicate it shortly. This paper intends to highlight the quinoline scaffold's potential for developing novel antileishmanial agents and provide a set of structural guidelines to help the research groups in the medicinal chemistry field perform more direct drug discovery and development programs. Thus, this review paper presents a thorough compilation of the most recent advances in the development of new quinoline-based antileishmanial agents, with a particular focus on structure-activity relationship studies that should be considerably useful for the future of the field.
Keywords: N-heterocycles; amastigotes; leishmania; promastigotes; quinolines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
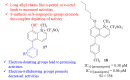
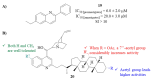
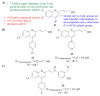
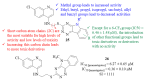




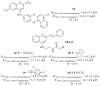
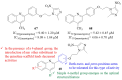
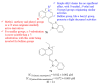
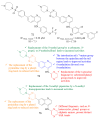
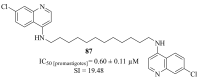
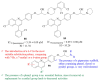
References
-
- World Health Organization . First WHO Report on Neglected Tropical Diseases: Working to Overcome the Global Impact of Neglected Tropical Diseases. World Health Organization; Geneva, Switzerland: 2010.
-
- World Health Organization Leishmaniasis. [(accessed on 11 February 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
Publication types
LinkOut - more resources
Full Text Sources

