Production and Immunogenicity Assessment of LTp50: An Escherichia coli-Made Chimeric Antigen Targeting S1- and S2-Epitopes from the SARS-CoV-2/BA.5 Spike Protein
- PMID: 38543088
- PMCID: PMC10975102
- DOI: 10.3390/ph17030302
Production and Immunogenicity Assessment of LTp50: An Escherichia coli-Made Chimeric Antigen Targeting S1- and S2-Epitopes from the SARS-CoV-2/BA.5 Spike Protein
Abstract
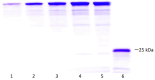
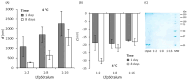
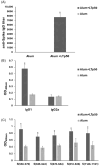
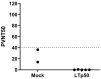
Subunit vaccines stand as a leading approach to expanding the current portfolio of vaccines to fight against COVID-19, seeking not only to lower costs but to achieve long-term immunity against variants of concern and have the main attributes that could overcome the limitations of the current vaccines. Herein a chimeric protein targeting S1 and S2 epitopes, called LTp50, was designed as a convenient approach to induce humoral responses against SARS-CoV-2. LTp50 was produced in recombinant Escherichia coli using a conventional pET vector, recovering the expected antigen in the insoluble fraction. LTp50 was purified by chromatography (purity > 90%). The solubilization and refolding stages helped to obtain a stable protein amenable for vaccine formulation. LTp50 was adsorbed onto alum, resulting in a stable formulation whose immunogenic properties were assessed in BALB/c mice. Significant humoral responses against the S protein (BA.5 variant) were detected in mice subjected to three subcutaneous doses (10 µg) of the LTp50/alum formulation. This study opens the path for the vaccine formulation optimization using additional adjuvants to advance in the development of a highly effective anti-COVID-19 vaccine directed against the antigenic regions of the S protein, which are less prone to mutations.
Keywords: COVID-19; built-in adjuvant; chimeric antigen; humoral response; linear epitopes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Chimeric Hepatitis B core virus-like particles harboring SARS-CoV2 epitope elicit a humoral immune response in mice.Microb Cell Fact. 2023 Feb 25;22(1):39. doi: 10.1186/s12934-023-02043-z. Microb Cell Fact. 2023. PMID: 36841778 Free PMC article.
-
Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection.Elife. 2022 Jan 24;11:e73490. doi: 10.7554/eLife.73490. Elife. 2022. PMID: 35072628 Free PMC article.
-
S Trimer Derived from SARS-CoV-2 B.1.351 and B.1.618 Induced Effective Immune Response against Multiple SARS-CoV-2 Variants.Vaccines (Basel). 2023 Jan 16;11(1):193. doi: 10.3390/vaccines11010193. Vaccines (Basel). 2023. PMID: 36680037 Free PMC article.
-
Preclinical Development of a Novel Epitope-based DNA Vaccine Candidate against SARS-CoV-2 and Evaluation of Immunogenicity in BALB/c Mice.AAPS PharmSciTech. 2024 Mar 12;25(3):60. doi: 10.1208/s12249-024-02778-x. AAPS PharmSciTech. 2024. PMID: 38472523
-
Development of Next Generation Vaccines against SARS-CoV-2 and Variants of Concern.Viruses. 2023 Feb 24;15(3):624. doi: 10.3390/v15030624. Viruses. 2023. PMID: 36992333 Free PMC article. Review.
Cited by
-
Layered Double Hydroxides (LDH) as Delivery Vehicles of a Chimeric Protein Carrying Epitopes from the Porcine Reproductive and Respiratory Syndrome Virus.Pharmaceutics. 2024 Jun 21;16(7):841. doi: 10.3390/pharmaceutics16070841. Pharmaceutics. 2024. PMID: 39065539 Free PMC article.
References
-
- WHO Coronavirus Disease (COVID-19) Pandemic. [(accessed on 10 June 2023)]. Available online: https://www.who.int/europe/emergencies/situations/covid-19.
-
- WHO WHO Chief Declares End to COVID-19 as a Global Health Emergency. [(accessed on 10 June 2023)]. Available online: https://news.un.org/en/story/2023/05/1136367.
-
- WHO WHO Coronavirus (COVID-19) Dashboard. [(accessed on 10 June 2023)]. Available online: https://covid19.who.int/
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

