Design of Etched- and Functionalized-Halloysite/Meloxicam Hybrids: A Tool for Enhancing Drug Solubility and Dissolution Rate
- PMID: 38543233
- PMCID: PMC10975188
- DOI: 10.3390/pharmaceutics16030338
Design of Etched- and Functionalized-Halloysite/Meloxicam Hybrids: A Tool for Enhancing Drug Solubility and Dissolution Rate
Abstract
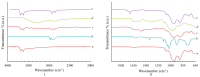
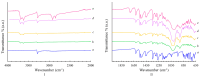
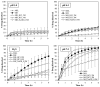
The study focuses on the synthesis and characterization of Meloxicam-halloysite nanotube (HNT) composites as a viable approach to enhance the solubility and dissolution rate of meloxicam, a poorly water-soluble drug (BCS class II). Meloxicam is loaded on commercial and modified halloysite (acidic and alkaline etching, or APTES and chitosan functionalization) via a solution method. Several techniques (XRPD, FT-IR, 13C solid-state NMR, SEM, EDS, TEM, DSC, TGA) are applied to characterize both HNTs and meloxicam-HNT systems. In all the investigated drug-clay hybrids, a high meloxicam loading of about 40 wt% is detected. The halloysite modification processes and the drug loading do not alter the structure and morphology of both meloxicam and halloysite nanotubes, which are in intimate contact in the composites. Weak drug-clay and drug-functionalizing agent interactions occur, involving the meloxicam amidic functional group. All the meloxicam-halloysite composites exhibit enhanced dissolution rates, as compared to meloxicam. The meloxicam-halloysite composite, functionalized with chitosan, showed the best performance both in water and in buffer at pH 7.5. The drug is completely released in 4-5 h in water and in less than 1 h in phosphate buffer. Notably, an equilibrium solubility of 13.7 ± 4.2 mg/L in distilled water at 21 °C is detected, and wettability dramatically increases, compared to the raw meloxicam. These promising results can be explained by the chitosan grafting on the outer surface of halloysite nanotubes, which provides increased specific surface area (100 m2/g) disposable for drug adsorption/desorption.
Keywords: dissolution tests; drug–nanoclay composites; halloysite nanotubes; meloxicam; poorly soluble drugs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Ainurofiq A., Putro D.S., Ramadhani D.A., Putra G.M., Do Espirito Santo L.D.C. A Review on Solubility Enhancement Methods for Poorly Water-Soluble Drugs. J. Rep. Pharm. Sci. 2021;10:137–147. doi: 10.4103/jrptps.JRPTPS_134_19. - DOI
LinkOut - more resources
Full Text Sources

