Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin
- PMID: 38543316
- PMCID: PMC10975060
- DOI: 10.3390/pharmaceutics16030423
Alginate and Chitosan-Based Delivery Systems for Improving the Bioavailability and Therapeutic Efficacy of Curcumin
Abstract
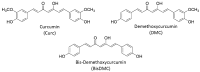
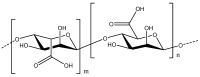
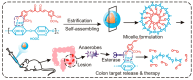
One of the major challenges in harnessing the therapeutic benefits of curcumin (an active ingredient from turmeric) is its poor bioavailability due to its short biological half-life. In this regard, nanoformulations have shown tremendous hope for improving the pharmacokinetic and therapeutic behavior of curcumin by altering its biological stability and bioavailability. Biopolymers, especially alginate and chitosan, have received special attention as excipients to prepare nanoformulations of curcumin due to their abundant availability, biocompatibility, and amicability to form different types of self-assembled structures and ease of undergoing chemical modifications. However, there are certain challenges, such as poor water solubility under physiological conditions and heterogeneity with regard to molecular weight and large-scale production of well-preserved nanostructures. Substantial advancement has been achieved towards overcoming these challenges by developing newer derivatives through a chemical modifications approach, and this has ascertained the suitability of alginate and chitosan as excipients for drug delivery systems (DDS). The present minireview briefly discusses curcumin and its limitation as a drug molecule, carbohydrates as DDS, and the recent developments related to the alginate and chitosan-based nanoformulations of curcumin. Special emphasis has been given to highlighting the impact of alginate and chitosan-based nanoformulations in improving the therapeutic efficacy and bioavailability of curcumin.
Keywords: alginate; chitosan; curcumin; drug delivery system; nano-formulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chitosan-based delivery systems for curcumin: A review of pharmacodynamic and pharmacokinetic aspects.J Cell Physiol. 2019 Aug;234(8):12325-12340. doi: 10.1002/jcp.28024. Epub 2019 Jan 30. J Cell Physiol. 2019. PMID: 30697728 Review.
-
Enhanced Water Dispersibility of Curcumin Encapsulated in Alginate-Polysorbate 80 Nano Particles and Bioavailability in Healthy Human Volunteers.Pharm Nanotechnol. 2019;7(1):39-56. doi: 10.2174/2211738507666190122121242. Pharm Nanotechnol. 2019. PMID: 30666922 Free PMC article.
-
Magnetic Alginate/Chitosan Nanoparticles for Targeted Delivery of Curcumin into Human Breast Cancer Cells.Nanomaterials (Basel). 2018 Nov 5;8(11):907. doi: 10.3390/nano8110907. Nanomaterials (Basel). 2018. PMID: 30400634 Free PMC article.
-
Cytotoxicity Effects of Curcumin Loaded on Chitosan Alginate Nanospheres on the KMBC-10 Spheroids Cell Line.Int J Nanomedicine. 2021 Jan 25;16:579-589. doi: 10.2147/IJN.S251056. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33531802 Free PMC article.
-
Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin.Expert Opin Drug Deliv. 2012 Nov;9(11):1347-64. doi: 10.1517/17425247.2012.724676. Epub 2012 Sep 13. Expert Opin Drug Deliv. 2012. PMID: 22971222 Review.
Cited by
-
Synergistic Effects of Natural Products and Mesenchymal Stem Cells in Osteoarthritis Treatment: A Narrative Review.Curr Issues Mol Biol. 2025 Jun 11;47(6):445. doi: 10.3390/cimb47060445. Curr Issues Mol Biol. 2025. PMID: 40699844 Free PMC article. Review.
-
Fabrication and Evaluation of Polyhydroxyalkanoate-Based Nanoparticles for Curcumin Delivery in Biomedical Applications.Molecules. 2025 Mar 8;30(6):1216. doi: 10.3390/molecules30061216. Molecules. 2025. PMID: 40141993 Free PMC article.
-
Lithium Coupled with C6-Carboxyl Improves the Efficacy of Oligoguluronate in DSS-Induced Ulcerative Colitis in C57BL/6J Mice.Mar Drugs. 2024 Dec 21;22(12):573. doi: 10.3390/md22120573. Mar Drugs. 2024. PMID: 39728147 Free PMC article.
References
-
- Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. - PubMed
-
- Kaur A. Historical background of usage of turmeric: A review. J. Pharmacogn. Phytochem. 2019;8:2769–2771.
-
- Giménez-Bastida J.A., Ávila-Gálvez M.Á., Carmena-Bargueno M., Pérez-Sánchez H., Espín J.C., González-Sarrías A. Physiologically relevant curcuminoids inhibit angiogenesis via VEGFR2 in human aortic endothelial cells. Food Chem. Toxicol. 2022;166:113254. doi: 10.1016/j.fct.2022.113254. - DOI - PubMed
-
- Sandur S.K., Pandey M.K., Sung B., Ahn K.S., Murakami A., Sethi G., Limtrakul P., Badmaev V., Aggarwal B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

