Sol-Gel Derived Gelatin-Bioactive Glass Nanocomposite Biomaterials Incorporating Calcium Chloride and Calcium Ethoxide
- PMID: 38543353
- PMCID: PMC10974492
- DOI: 10.3390/polym16060747
Sol-Gel Derived Gelatin-Bioactive Glass Nanocomposite Biomaterials Incorporating Calcium Chloride and Calcium Ethoxide
Abstract
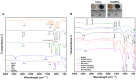
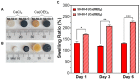
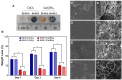
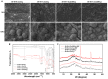
Calcium-containing organic-inorganic nanocomposites play an essential role in developing bioactive bone biomaterials. Ideally, bone substitute materials should mimic the organic-inorganic composition of bone. In this study, the roles of calcium chloride (CaCl2) and calcium ethoxide (Ca(OEt)2) were evaluated for the development of sol-gel-derived organic-inorganic biomaterials composed of gelatin, bioactive glass (BG) and multiwall carbon nanotubes (MWCNTs) to create nanocomposites that mimic the elemental composition of bone. Nanocomposites composed of either CaCl2 or Ca(OEt)2 were chemically different but presented uniform elemental distribution. The role of calcium sources in the matrix of the nanocomposites played a major role in the swelling and degradation properties of biomaterials as a function of time, as well as the resulting porous properties of the nanocomposites. Regardless of the calcium source type, biomineralization in simulated body fluid and favorable cell attachment were promoted on the nanocomposites. 10T1/2 cell viability studies using standard media (DMEM with 5% FBS) and conditioned media showed that Ca(OEt)2-based nanocomposites seemed more favorable biomaterials. Collectively, our study demonstrated that CaCl2 and Ca(OEt)2 could be used to prepare sol-gel-derived gelatin-BG-MWCNT nanocomposites, which have the potential to function as bone biomaterials.
Keywords: bioactivity; bone biomaterial; calcium; gelatin–bioactive glass–MWCNT biomaterials; nanocomposites; sol-gel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Bioactive and electrically conductive GelMA-BG-MWCNT nanocomposite hydrogel bone biomaterials.Biomater Adv. 2023 Nov;154:213616. doi: 10.1016/j.bioadv.2023.213616. Epub 2023 Sep 4. Biomater Adv. 2023. PMID: 37708668
-
Osteogenic Differentiation Potential of iMSCs on GelMA-BG-MWCNT Nanocomposite Hydrogels.Biomimetics (Basel). 2024 Jun 3;9(6):338. doi: 10.3390/biomimetics9060338. Biomimetics (Basel). 2024. PMID: 38921218 Free PMC article.
-
Synthesis and electrospinning of ε-polycaprolactone-bioactive glass hybrid biomaterials via a sol-gel process.Langmuir. 2010 Dec 7;26(23):18340-8. doi: 10.1021/la102845k. Epub 2010 Nov 4. Langmuir. 2010. PMID: 21050002
-
Composite Biomaterials Based on Sol-Gel Mesoporous Silicate Glasses: A Review.Bioengineering (Basel). 2017 Feb 23;4(1):15. doi: 10.3390/bioengineering4010015. Bioengineering (Basel). 2017. PMID: 28952496 Free PMC article. Review.
-
A comprehensive review on nanocomposite biomaterials based on gelatin for bone tissue engineering.Int J Biol Macromol. 2024 Jan;254(Pt 1):127556. doi: 10.1016/j.ijbiomac.2023.127556. Epub 2023 Oct 25. Int J Biol Macromol. 2024. PMID: 37884249 Review.
References
-
- Tallia F., Ting H.-K., Page S.J., Clark J.P., Li S., Sang T., Russo L., Stevens M.M., Hanna J.V., Jones J.R. Bioactive, Degradable and Tough Hybrids Through Calcium and Phosphate Incorporation. Front. Mater. 2022;9:901196. doi: 10.3389/fmats.2022.901196. - DOI
-
- Hench L.L. Bioceramics. J. Am. Ceram. Soc. 2005;81:1705–1728. doi: 10.1111/j.1151-2916.1998.tb02540.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

