Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens
- PMID: 38543492
- PMCID: PMC10972368
- DOI: 10.3390/microorganisms12030441
Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens
Abstract
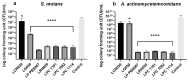
The microbiota in the oral cavity has a strict connection to its host. Its imbalance may determine oral diseases and can also have an impact on the systemic health. Probiotic strains may help in the restoration of a balanced condition. For this purpose, we screened the antibacterial and antiadhesive activities of many viable probiotic strains (Lactobacillus acidophilus PBS066, Lactobacillus crispatus LCR030, Lactobacillus gasseri LG050, Lactiplantibacillus plantarum PBS067, Limosilactobacillus reuteri PBS072, Lacticaseibacillus rhamnosus LRH020, Bifidobacterium animalis subsp. lactis BL050, Lacticaseibacillus paracasei LPC 1101, L. paracasei LPC 1082, and L. paracasei LPC 1114) against two main oral pathogens, Streptococcus mutans and Aggregatibacter actinomycetemcomitans, involved in dental caries and periodontal disease development and progression. Considering both the agar overlay preventive and treatment models, seven probiotics determined greater inhibition zones against the tested pathogens. This behavior was further analyzed by the plate count method and scanning electron microscope imaging. L. plantarum PBS067, L. rhamnosus LRH020, L. paracasei LPC 1101, L. paracasei LPC 1082, and L. paracasei LPC 1114 prevent the growth and adhesion of oral pathogens in a strain-specific manner (p < 0.0001). These probiotics might be considered as an alternative effective adjuvant to improve oral and systemic well-being for future personalized treatments.
Keywords: dysbiosis; host interaction; infection; microbiota; oral; pathogens; probiotics.
Conflict of interest statement
Authors D.F.S. and P.M. were employed by the company Synbalance Srl. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens.Lett Appl Microbiol. 2020 Apr;70(4):310-317. doi: 10.1111/lam.13275. Epub 2020 Feb 11. Lett Appl Microbiol. 2020. PMID: 31955445
-
Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial.Nutrients. 2024 Jan 30;16(3):402. doi: 10.3390/nu16030402. Nutrients. 2024. PMID: 38337685 Free PMC article. Clinical Trial.
-
Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens.Int J Mol Sci. 2023 Jan 10;24(2):1323. doi: 10.3390/ijms24021323. Int J Mol Sci. 2023. PMID: 36674840 Free PMC article.
-
Lactobacilli Infection Case Reports in the Last Three Years and Safety Implications.Nutrients. 2022 Mar 11;14(6):1178. doi: 10.3390/nu14061178. Nutrients. 2022. PMID: 35334835 Free PMC article. Review.
-
Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections.Int J Antimicrob Agents. 2013 Dec;42(6):475-81. doi: 10.1016/j.ijantimicag.2013.08.003. Epub 2013 Sep 7. Int J Antimicrob Agents. 2013. PMID: 24071026 Review.
Cited by
-
Editorial for Oral Microbes and Human Health.Microorganisms. 2025 Apr 16;13(4):922. doi: 10.3390/microorganisms13040922. Microorganisms. 2025. PMID: 40284758 Free PMC article.
-
Evaluating the Impact of Kefir Consumption on Dental Caries and Periodontal Disease: A Narrative Review.Dent J (Basel). 2025 Feb 18;13(2):86. doi: 10.3390/dj13020086. Dent J (Basel). 2025. PMID: 39996960 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

