Active Microbiota of Penaeus stylirostris Larvae: Partially Shaped via Vertical and Horizontal Transmissions and Larval Ontogeny
- PMID: 38543660
- PMCID: PMC10976216
- DOI: 10.3390/microorganisms12030608
Active Microbiota of Penaeus stylirostris Larvae: Partially Shaped via Vertical and Horizontal Transmissions and Larval Ontogeny
Abstract
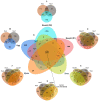
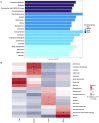
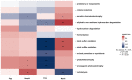
During their entire lifecycle, mariculture animals are farmed in water that contains various microorganisms with which they are in close associations. Microbial exchanges between the animals and their surrounding water can occur. However, little is known about the interactions between shrimp larvae and water, and more especially, about larval bacterial selection and microbiota modulation across ontogeny. To address this gap, using HiSeq sequencing targeting the V4 region of the 16S rRNA molecule, we investigated the active prokaryotic diversity and structure of healthy Penaeus stylirostris larvae and seawater. Comparisons between different larval stages revealed evidence of stage-specific microbiotas and biomarkers, a core microbiota common to all stages, and shared taxa between successive stages, suggesting vertical transmission of bacterial taxa. Comparisons between stage-specific microbiotas and core microbiotas with water storages highlighted that many taxa associated with the larvae were originally present in the natural seawater, underlining horizontal transmission of bacteria from water to larvae. As some of these lineages became active at specific larval stages, we suggest that larvae were able to modulate their microbiota. This study provides insight into larvae-microbiota interactions at the larval stage scale.
Keywords: active microbiota; biomarkers; larvae; shrimp; shrimp ontogeny; specific microbiota; taxa transmission.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Zheng Y., Yu M., Liu J., Qiao Y., Wang L., Li Z., Zhang X.H., Yu M. Bacterial Community Associated with Healthy and Diseased Pacific White Shrimp (Litopenaeus vannamei) Larvae and Rearing Water across Different Growth Stages. Front. Microbiol. 2017;8:244804. doi: 10.3389/fmicb.2017.01362. - DOI - PMC - PubMed
-
- Sun F., Wang Y., Wang C., Zhang L., Tu K., Zheng Z. Insights into the Intestinal Microbiota of Several Aquatic Organisms and Association with the Surrounding Environment. Aquaculture. 2019;507:196–202. doi: 10.1016/j.aquaculture.2019.04.026. - DOI
-
- Gomez-Gil B., Roque A., Turnbull J.F. The Use and Selection of Probiotic Bacteria for Use in the Culture of Larval Aquatic Organisms. Aquaculture. 2000;191:259–270. doi: 10.1016/S0044-8486(00)00431-2. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

