Preclinical Antiviral and Safety Profiling of the HBV RNA Destabilizer AB-161
- PMID: 38543689
- PMCID: PMC10975527
- DOI: 10.3390/v16030323
Preclinical Antiviral and Safety Profiling of the HBV RNA Destabilizer AB-161
Abstract
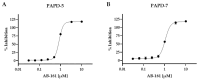
HBV RNA destabilizers are a class of small-molecule compounds that target the noncanonical poly(A) RNA polymerases PAPD5 and PAPD7, resulting in HBV RNA degradation and the suppression of viral proteins including the hepatitis B surface antigen (HBsAg). AB-161 is a next-generation HBV RNA destabilizer with potent antiviral activity, inhibiting HBsAg expressed from cccDNA and integrated HBV DNA in HBV cell-based models. AB-161 exhibits broad HBV genotype coverage, maintains activity against variants resistant to nucleoside analogs, and shows additive effects on HBV replication when combined with other classes of HBV inhibitors. In AAV-HBV-transduced mice, the dose-dependent reduction of HBsAg correlated with concentrations of AB-161 in the liver reaching above its effective concentration mediating 90% inhibition (EC90), compared to concentrations in plasma which were substantially below its EC90, indicating that high liver exposure drives antiviral activities. In preclinical 13-week safety studies, minor non-adverse delays in sensory nerve conductance velocity were noted in the high-dose groups in rats and dogs. However, all nerve conduction metrics remained within physiologically normal ranges, with no neurobehavioral or histopathological findings. Despite the improved neurotoxicity profile, microscopic findings associated with male reproductive toxicity were detected in dogs, which subsequently led to the discontinuation of AB-161's clinical development.
Keywords: HBV RNA; HBV RNA destabilizer; PAPD5; PAPD7.
Conflict of interest statement
All authors are or were employed by Arbutus Biopharma and may hold company stock.
Figures
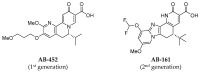

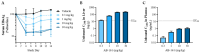


References
-
- World Health Organization Global Hepatitis Report. 2017. [(accessed on 13 February 2024)]. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

