HBcAb Positivity as a Risk Factor for Missing HIV RNA Undetectability after the 3TC+DTG Switch
- PMID: 38543714
- PMCID: PMC10974397
- DOI: 10.3390/v16030348
HBcAb Positivity as a Risk Factor for Missing HIV RNA Undetectability after the 3TC+DTG Switch
Abstract
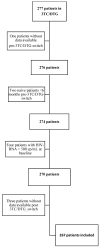
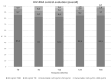
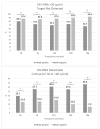
Hepatitis B Core antibody (HBcAb) positivity is the surrogate marker of hepatitis B occult infection. This condition is not a contraindication for switching to two-drug (2DR) antiretroviral therapy; however, the removal of tenofovir may contribute to poor control of HBV replication. A multicentre retrospective cohort study investigated the impact of HBcAb positivity on HIV control in patients switching to a 2DR with Lamivudine and Dolutegravir (3TC-DTG). In this study, a comparison analysis was conducted between HBcAb-positive and -negative PLWH regarding HIV-RNA suppression, considering: (1): Target Not Detected (TND) < 20 cp/mL; (2) Target Detected (TD) < 20 cp/mL; and (3) Detectable > 20 cp/mL and <50 cp/mL and >50 copies/mL. A total of 267 patients on 2DR with 3TC-DTG were included. In comparison to HBcAb-negative, HBcAb-positive patients were older (45 years [35-54]) and had a lower CD4+ nadir (248 vs. 349 cells/mmc, p = 0.007). No difference in the maintenance of virological suppression was present in the two groups of patients before the switch. Although no patient had an HIV-RNA > 20 cp/mL after the switch, significantly fewer HBcAb-positive compared with -negative subjects resulted in TND at 12, 24, and 36 months after the switch: 52 (69.3%) versus 164 (85.4%), p = 0.004, 50 [72.5%] versus 143 [89.9%], p = 0.001, and 30 [66.7%] versus 90 [92.8%], p = 0.001, respectively. HBcAb positivity is associated with an increased risk of suboptimal HIV suppression during the 36 months after 3TC/DTG simplification. This finding reinforces the relevance of the OBI condition in PLWH and raises the issue of careful virological monitoring of such cases.
Keywords: HBcAb+; HIV/HBV; OBI.
Conflict of interest statement
The authors have no conflicts of interest to report. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Baldin G., Ciccullo A., Rusconi S., Capetti A., Sterrantino G., Colafigli M., D’Ettorre G., Giacometti A., Cossu M.V., Borghetti A., et al. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int. J. Antimicrob. Agents. 2019;54:728–734. doi: 10.1016/j.ijantimicag.2019.09.002. - DOI - PubMed
-
- Li J.Z., Sax P.E., Marconi V.C., Fajnzylber J., Berzins B., Nyaku A.N., Fichtenbaum C.J., Wilkin T., Benson C.A., Koletar S.L., et al. No Significant Changes to Residual Viremia After Switch to Dolutegravir and Lamivudine in a Randomized Trial. Open Forum Infect. Dis. 2019;6:ofz056. doi: 10.1093/ofid/ofz056. - DOI - PMC - PubMed
-
- Trujillo-Rodríguez M., Muñoz-Muela E., Serna-Gallego A., Milanés-Guisado Y., Praena-Fernández J.M., Álvarez-Ríos A.I., Herrera-Hidalgo L., Domínguez M., Lozano C., Romero-Vazquez G., et al. Immunological and inflammatory changes after simplifying to dual therapy in virologically suppressed HIV-infected patients through week 96 in a randomized trial. Clin. Microbiol. Infect. 2022;28:1151.e9–1151.e16. doi: 10.1016/j.cmi.2022.02.041. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous