IFNα Subtypes in HIV Infection and Immunity
- PMID: 38543729
- PMCID: PMC10975235
- DOI: 10.3390/v16030364
IFNα Subtypes in HIV Infection and Immunity
Abstract
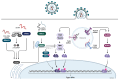
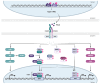
Type I interferons (IFN), immediately triggered following most viral infections, play a pivotal role in direct antiviral immunity and act as a bridge between innate and adaptive immune responses. However, numerous viruses have evolved evasion strategies against IFN responses, prompting the exploration of therapeutic alternatives for viral infections. Within the type I IFN family, 12 IFNα subtypes exist, all binding to the same receptor but displaying significant variations in their biological activities. Currently, clinical treatments for chronic virus infections predominantly rely on a single IFNα subtype (IFNα2a/b). However, the efficacy of this therapeutic treatment is relatively limited, particularly in the context of Human Immunodeficiency Virus (HIV) infection. Recent investigations have delved into alternative IFNα subtypes, identifying certain subtypes as highly potent, and their antiviral and immunomodulatory properties have been extensively characterized. This review consolidates recent findings on the roles of individual IFNα subtypes during HIV and Simian Immunodeficiency Virus (SIV) infections. It encompasses their induction in the context of HIV/SIV infection, their antiretroviral activity, and the diverse regulation of the immune response against HIV by distinct IFNα subtypes. These insights may pave the way for innovative strategies in HIV cure or functional cure studies.
Keywords: HIV; IFNα subtypes; immunotherapy; type I IFNs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
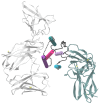
References
-
- Trilling M., Bellora N., Rutkowski A.J., de Graaf M., Dickinson P., Robertson K., da Costa O.P., Ghazal P., Friedel C.C., Alba M.M., et al. Deciphering the modulation of gene expression by type I and II interferons combining 4sU-tagging, translational arrest and in silico promoter analysis. Nucleic Acids Res. 2013;41:8107–8125. doi: 10.1093/nar/gkt589. - DOI - PMC - PubMed
-
- Megger D.A., Philipp J., Le-Trilling V.T.K., Sitek B., Trilling M. Deciphering of the Human Interferon-Regulated Proteome by Mass Spectrometry-Based Quantitative Analysis Reveals Extent and Dynamics of Protein Induction and Repression. Front. Immunol. 2017;8:1139. doi: 10.3389/fimmu.2017.01139. - DOI - PMC - PubMed
-
- Sertznig H., Roesmann F., Wilhelm A., Heininger D., Bleekmann B., Elsner C., Santiago M., Schuhenn J., Karakoese Z., Benatzy Y., et al. SRSF1 acts as an IFN-I-regulated cellular dependency factor decisively affecting HIV-1 post-integration steps. Front. Immunol. 2022;13:935800. doi: 10.3389/fimmu.2022.935800. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

